
Anticoagulation therapy in Australia
Introduction
Anticoagulant therapy is prescribed to a variety of patients for a variety of clinical indications (1-5). For example, vitamin K antagonists (VKAs), such as warfarin, remain one of the most commonly utilised oral anticoagulants and are broadly used for: treatment of venous thromboembolism (VTE) including deep vein thrombosis (DVT) and pulmonary embolism (PE), stroke prophylaxis in atrial fibrillation (AF) and to reduce the risk of prosthetic heart valve thrombosis and thromboembolism (1-3,6). Heparin moieties, for example either as unfractionated (UH), or as a low molecular weight heparin (LMWH), represent other commonly prescribed anticoagulants, albeit given parentally (either intravenous or subcutaneous) (1-4,6). More recently, the availability of direct inhibitors of either thrombin (factor IIa) or factor Xa (FXa), have revolutionised the landscape of oral anticoagulant therapy and given rise to the concept of ‘direct oral anticoagulants’ (or DOACs) (1-3,5,6). In this narrative review, we briefly outline the anticoagulants available in Australia, their relative strengths and weaknesses, and the ever-changing local landscape with regards to anticoagulation therapy.
Heparin anticoagulation therapy
As noted above, heparin therapy is reflected by different options, broadly reflective of UF, LMWH, and in some cases heparinoids and heparin-like molecules that indirectly or directly inhibit either thrombin (FIIa) or FXa. The main advantages and disadvantages of heparin therapy are provided in Table 1.
Table 1
| Anticoagulant agent | Anticoagulant activity | Summary of current clinical indication(s) | Laboratory tests used to monitor and/or assess activity? | Strengths/benefits/advantages | Limitations/weaknesses/disadvantages |
|---|---|---|---|---|---|
| Unfractionated heparin | Anti-thrombin (FIIa) activity (and to lesser extent anti-FXa, FIXa and FXIa activity) | Prophylaxis and treatment of thromboembolic disorders [e.g., thrombophlebitis, pulmonary embolism, deep vein thrombosis (DVT)] and occlusive vascular disease. Also used to prevent thromboembolic complications arising from cardiac and vascular surgery, frostbite, dialysis and other perfusion procedures | Monitored regularly using an APTT (e.g., 1.5–2.5× baseline APTT, or equivalent to 0.3–0.7 U heparin by anti-FXa assay). Monitor platelet count to assess/mitigate risk of heparin induced thrombocytopenia (thrombosis) syndrome (HIT). Chromogenic anti-FXa assay occasionally used to monitor patients (e.g., in lupus anticoagulant positive or FXII deficient patients). Thrombin time occasionally used to assess presence or absence of heparin | Fast acting and clinically effective anticoagulant. Low cost. Antidotes available (e.g., protamine sulphate) | Parental administration. Requires regular monitoring (APTT or anti-FXa). Narrow therapeutic window. Inter-individual pharmacodynamic variability. Risk of HIT. Requires monitoring of platelet count. Potential for over-dose or under-dosing if error in administration, or if not monitored |
| Low molecular weight heparins (e.g., Clexane, Fragmin) | Direct anti-FXa activity (and to lesser extent anti-thrombin) | Prevention of venous thromboembolism (surgical, acutely unwell medical patients), extracorporeal thrombosis during haemodialysis. Treatment of established DVT and acute coronary syndromes (ACS) | Monitoring is not usually required. If desired, use chromogenic anti-FXa assay (e.g., therapeutic target of 0.6–1.0 U/mL) | Fast acting and clinically effective anticoagulant. Does not require regular monitoring except in select cases. Reduced risk of HIT compared to unfractionated heparin | Parental administration. Some risk of HIT. More expensive than unfractionated heparin. Some individuals (e.g., pregnancy, pediatric patients, those at extremes of body weight, severely disturbed hepatic function, significant renal failure) may require monitoring by laboratory tests |
Table updated from (
The main advantages are the low cost of therapy, rapid onset of action, clinical efficacy, and that (for UH at least) an antidote is readily available in the case of over-dose or need for reversal in the setting of bleeding or urgent surgery.
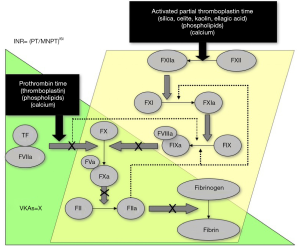
One easily identified limitation of heparin therapy is that it needs to be administered intravenously or subcutaneously to achieve anticoagulation, either regularly administered (1–2× per day) or by continuous infusion (UH). This may be inconvenient or undesired. Heparin therapy may also require laboratory monitoring, either by use of an activated partial thromboplastin (APTT) assay (for UH), or by a chromogenic anti-FXa assay (LMWH; sometimes UH) (Table 1, Figures 1 and 2). This adds cost and complexity to anticoagulant therapy management. Testing and clinical outcomes may also be compromised by poorly selected heparin therapeutic ranges, based on misunderstandings or limited assessment of reagent sensitivities (4). It is usual also to monitor platelet counts, as there is a small but significant risk of developing heparin induced thrombocytopenia (HIT), which may have significant adverse clinical sequelae, such as thrombosis and organ damage (7,8). This is known as ‘pathological HIT’ or HIT with thrombosis, sometimes abbreviated as HITT.
VKA anticoagulation therapy
Treatment with VKAs such as warfarin also carries both advantages and disadvantages (Table 2). VKAs represent cheap and clinically effective anticoagulants and are administered orally. There are antidotes available in the case of over-dose or therapy reversal (e.g., urgent surgery).
Table 2
| Anticoagulant agent | Anticoagulant activity | Summary of current clinical indication(s) | Laboratory tests used to monitor and/or assess activity? | Strengths/benefits/advantages | Limitations/weaknesses/disadvantages |
|---|---|---|---|---|---|
| Vitamin K antagonists (VKA) or coumarins (e.g., warfarin) | Affect vitamin K sensitive factors (i.e., II, VII, IX & X) | Prophylaxis and/or treatment of venous thrombosis and its extension and pulmonary embolism. Prophylaxis and/or treatment of the thromboembolic complications associated with atrial fibrillation. Prevention of thromboembolic events in patients with prosthetic heart valves. Use as an adjunct in the treatment of coronary occlusion | Monitor regularly using the prothrombin time (PT) converted to an international normalized ratio (INR). INR =2.0–3.0 for most indications (2.5–3.5 or even values <2 may be used for specific indications) | Clinically effective anticoagulants. Oral administration. Low cost. Antidotes available (e.g., vitamin K, prothrombin complex concentrates, fresh frozen plasma). Monitoring means regular checks for patient compliance. Point of care testing/self-monitoring is available in some countries | Slow acting. Requires regular monitoring (narrow therapeutic window; many drug and food interactions; pharmacogenetic influences; large inter-individual variation). High risk of serious adverse effects related to inappropriate dosing |
Table updated from (
Among the limitations, VKAs are slow acting, there is a large inter-individual variation in anticoagulant response, and there are many drug and food interactions. These limitations mandate initial therapy with another agent (usually heparin), and regular laboratory monitoring, most typically using the prothrombin time (PT), converted to an international normalised ratio (INR) (Figure 1). This is problematic for many reasons, and not only for the regular (inconvenient, undesired, time consuming) venous punctures required for blood sampling. The INR, as a mathematical calculation of the laboratory test the PT, itself poses many problems, including limited accuracy (9-11).
DOACs
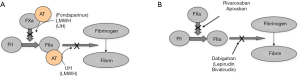
Despite the clinical efficacy of anticoagulant agents such as heparin and VKAs, their limitations have driven continued advances in anticoagulant therapy, most recently epitomised by the release of several direct acting inhibitors of FIIa or FXa, and now generally known as DOACs. These include the agents dabigatran (anti-FIIa) and in Australia apixaban and rivaroxaban (both anti-FXa agents). Additional DOACs are available in other countries, including edoxaban and betrixaban (both anti-FXa agents). The clinical indications for the DOACs are similar to those of heparin and/or VKAs, and also similar to one another (Table 3). Such indications may be somewhat different in other countries, depending on local regulatory approvals/clearances.
Table 3
| Anticoagulant agent | Anticoagulant activity | Summary of current clinical indication(s) | Laboratory tests used to monitor and/or assess activity? | Strengths/benefits/advantages | Limitations/weaknesses/disadvantages |
|---|---|---|---|---|---|
| Dabigatran | Anti-thrombin (FIIa) | Prevention of venous thromboembolic events in adult patients who have undergone major orthopaedic surgery of the lower limb (elective total hip or knee replacement. Prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation and at least one additional risk factor for stroke. Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and for the prevention of recurrent DVT and PE in adults* | Laboratory monitoring of anticoagulant therapy is not required. If desired (e.g., urgent surgery), dabigatran levels can be measured using a direct thrombin inhibitor assay. Laboratory tests to assess liver and renal function are recommended, as is some regular assessment | Clinically effective anticoagulant. Oral administration. Fast acting. Does not require laboratory monitoring. Antidote available (idarucizumab) for select indications | More expensive agent than heparin/VKAs. Potential for over-dose or under-dose if patient non-compliant, with compliance difficult to assess (no laboratory monitoring). Antidote is expensive (>$5,000/treatment) |
| Rivaroxaban | Anti-FXa | Prevention of venous thromboembolism (VTE) in adult patients who have undergone major orthopaedic surgery of the lower limbs (elective total hip replacement, treatment for up to 5 weeks; elective total knee replacement, treatment for up to 2 weeks). Prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation and at least one additional risk factor for stroke. Treatment of DVT and PE and for the prevention of recurrent DVT and PE | Laboratory monitoring of anticoagulant therapy is not required. If desired (e.g., urgent surgery), rivaroxaban levels can be measured using a chromogenic anti-FXa assay. Laboratory tests to assess liver and renal function are recommended, as is some regular assessment | Clinically effective anticoagulant. Oral administration. Fast acting. Does not require laboratory monitoring. Antidote pending (andexanet) | More expensive agent than heparin/VKAs. Potential for over-dose or under-dose if patient non-compliant, with compliance difficult to assess (no laboratory monitoring). Once available, antidote is expected to be expensive |
| Apixaban | Anti-FXa | Prevention of VTE in adult patients who have undergone elective total hip or total knee replacement surgery. Prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation and at least one additional risk factor for stroke. Treatment of DVT and PE in adult patients. Prevention of recurrent DVT and PE in adult patients | Laboratory monitoring of anticoagulant therapy is not required. If desired (e.g., urgent surgery), apixaban levels can be measured using a chromogenic anti-FXa assay. Laboratory tests to assess liver and renal function are recommended, as is some regular assessment | Clinically effective anticoagulant. Oral administration. Fast acting. Does not require laboratory monitoring. Antidote pending (andexanet) | More expensive agent than heparin/VKAs. Potential for over-dose or under-dose if patient non-compliant, with compliance difficult to assess (no laboratory monitoring). Once available, antidote is expected to be expensive |
Table modified from (
In terms of advantages, all the DOACs have proven efficacy and safety. Indeed, studies show either similar or superior outcomes with the DOACs in comparison to comparator drugs (12-14). The DOACs were also developed and are marketed as not requiring laboratory monitoring, and so are far more convenient than alternative classical anticoagulant agents. Testing, if required, van be performed (5,15). Idarucizumab is available for reversal of dabigatran in the setting of urgent surgery or life-threatening bleeding (16); however, specific antidotes for the Factor Xa inhibitors are not yet available in Australia, although under clinical trial.
In terms of disadvantages, DOACs are more expensive than heparin or VKAs (Figure 3), although cost-benefit data may suggest indirect cost savings to the health system with their implementation (e.g., reduction in bleeding and hence less hospital admissions and no requirement for routine laboratory monitoring). Since routine laboratory monitoring is not undertaken, there is a potential risk of both over-dosing and under-dosing, with consequent increased risk of bleeding or thrombosis, respectively, in particular in non-compliant patients or those with relevant co-morbidities. Indeed, compliance is a big issue with the DOACs. With VKAs, a missed dose could be adjusted for. With DOACs, a missed dose leads to lack of anticoagulant cover for 12–24 hours depending on the agent. In addition, should patients miss a dose, they may be tempted to take a double dose the next time, which would place them at higher risk for a bleeding event. Although antidotes are available or imminent for DOACs, they are very expensive relative to those used for heparin and VKAs. Although regular monitoring of anticoagulant therapy is not needed, ongoing risk assessment of renal or liver dysfunction (and monitoring of appropriate parameters) is required because of the risk of drug accumulation in these settings which may result in an increased risk of bleeding.
Other anticoagulant agents
In Australia, several other anticoagulants are available for various clinical indications, as summarised in Table 4 (2). These drugs tend to have fairly restricted applications in hospital settings, and are not broadly available for community use. They tend to need close monitoring and are also relatively expensive to use (Figure 2, Table 4).
Table 4
| Anticoagulant agent | Anticoagulant activity | Summary of current clinical indication(s) | Laboratory tests used to monitor and/or assess activity? | Strengths/benefits/advantages | Limitations/weaknesses/disadvantages |
|---|---|---|---|---|---|
| Fondaparinux | Indirect FXa inhibitor | Prevention of venous thromboembolism (VTE) in major orthopaedic surgery, at risk abdominal surgery patients. Treatment of acute DVT, PE | Not monitored. Anti-Xa assay may be used to identify if activity is present | 100% adsorption by s.c. administration. Laboratory monitoring not required. Very low risk of developing HIT. Chemical synthesis (i.e., no risk of viral contamination) | Parental agent. Greater risk of bleeding especially in the elderly. Contraindicated in patients with severe kidney disease and thrombocytopenia. No antidote available |
| Lepirudin (hirudin) | Direct thrombin inhibitor | Acute heparin induced thrombocytopenia type II patients with thrombocytopenia, thromboembolic complications | Monitor prolonged usage using APTT (1.5–2.5× baseline APTT). Other tests sensitive to direct thrombin inhibitors might play additional roles in the future (e.g., ECT) | No risk of developing HIT. Inhibition of clot- and fibrin-bound thrombin | Parental agent. Very narrow therapeutic range (i.e., 0.6–1.0 ìg/mL). Development of anti-lepirudin antibodies in 40–70% of patients. Strict laboratory monitoring required (especially in specific settings such as decreased renal function). No definitive monitoring test available (e.g., APTT shows non-linear dose-response) |
| Bivalirudin (oligopeptide of hirudin) | Direct thrombin inhibitor | For use as an anticoagulant in the treatment of patients with moderate to high risk ACS (unstable angina/non-ST segment elevation myocardial infarction (UA/NSTEMI) who are undergoing early invasive management, and in patients undergoing PCI. (NB: intended for use with aspirin; a P2Y12 antagonist (e.g., clopidogrel or ticlopidine) may be used in addition to aspirin). For use as potential alternative to heparin in patients with, or at risk of, HIT/T | May be monitored using the activated clotting time (ACT) if required | No risk of developing HIT. Safer alternative to heparin during PCI. Inhibition of clot- and fibrin-bound thrombin as well as thrombin-mediated platelet activation and aggregation. Highly predictable antithrombotic response (rapid onset of action, short half-life and no significant binding to plasma proteins) | Parental agent. Limited indications (i.e., UA/STEMI and related therapeutic procedures) |
Table modified from (
Discussion
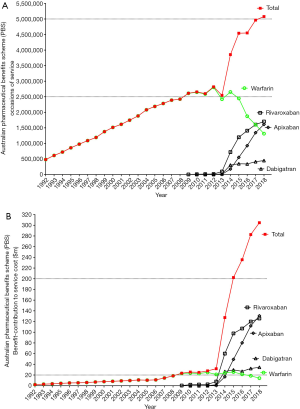
Because of apparent benefits, DOACs are becoming increasingly prescribed by clinicians and utilised by patients. Indeed, local Australian prescription data shows that DOACs have now replaced VKA therapy as the most often prescribed anticoagulant drugs (Figure 3). The clear ongoing reduction in warfarin prescribing contrasts the steady increase in prescriptions for all the DOACs, although most notably for the anti-activated factor X (FXa) agents (rivaroxaban and apixaban). The preferential uptake of the anti-FXa agent rivaroxaban and apixaban over dabigatran may be partly related to the Australian setting, where the price of dabigatran is not subsidised by the government for treatment of DVT/PE. What this data also seems to show is that the total number of prescriptions for all oral anticoagulants (warfarin, apixaban, rivaroxaban and dabigatran) has also increased substantially over the data collection period. However, there are limitations in the direct comparability of this data, given this details prescription counts for each drug, with each being available in several forms, which may not be ‘analogous’ (e.g., warfarin is available in 1, 2, 3 and 5 mg tablets; rivaroxaban is available in 10, 15 or 20 mg tablets, of various pack sizes). Nevertheless, these trends appear to evidence that DOACs are not only replacing warfarin, but are also potentially being increasingly prescribed in previously anticoagulant-naïve patients. Another possible contributor to these trends is that more patients are remaining on long-term VTE prophylaxis with reduced dose anti-FXa agents. The available data does not provide any detail to offer any reassurance or otherwise regarding the appropriateness of these prescriptions, although because the risk/benefit ratio of DOACs is typically identified as being superior to VKAs, clinicians may be more comfortable starting anticoagulant therapy in some patients previously deemed unsuitable for VKA, or in extending their anticoagulation for longer/indefinitely at reduced prophylactic doses of anti-FXa agents.
Of importance is that any list of drug indications is usually counterbalanced by a sometimes longer list of ‘contraindications’, which for the oral anticoagulants is summarised in Table 5. Accordingly, although these are considered ‘safe drugs’, no drug should be prescribed without due consideration of associated risks in each individual patient.
Table 5
| Anticoagulant agent | Not indicated/contraindicated |
|---|---|
| Vitamin K antagonists (VKA) or coumarins (e.g., warfarin) | Not indicated in patients with lone atrial fibrillation, i.e., CHADS2 or CHA2DS2-VASc score <2 |
| If risk of major bleeding (estimated by HAS-BLED or HEMORR2HAGES scores) exceeds the benefits of anticoagulation (e.g., stroke prevention in AF, recurrence of VTE) | |
| Otherwise contraindicated in following circumstances: | |
| Pregnancy | |
| Haemorrhagic tendencies or blood dyscrasias (e.g., platelet count <50×109/L) | |
| Recent or contemplated surgery of high bleeding risk | |
| Active or recent bleeding (e.g., from GI or GU) or history of retinal or cerebrovascular haemorrhage | |
| Known large esophageal varices, decompensated liver disease | |
| Malignant hypertension | |
| Inadequate access to laboratory facilities or POC devices | |
| Non-adherence to treatment or monitoring | |
| Dabigatran | Contraindicated in patients with following circumstances: |
| Known hypersensitivity | |
| Creatinine clearance <30 mL/min | |
| Clinically significant active bleeding | |
| Significant inherited or acquired bleeding disorder | |
| Hepatic disease with coagulopathy (Child-Pugh C) | |
| Organ lesions at risk of bleeding including intracranial haemorrhage in previous 6 months | |
| Indwelling spinal or epidural catheter and during the first six hours after removal | |
| Mechanical heart valve | |
| Pregnancy or breastfeeding mother | |
| Concomitant treatment with any other anticoagulants unless transitioning between anticoagulants | |
| GI haemorrhage within the past year unless the cause has been permanently eliminated, e.g., by surgery | |
| Concomitant treatment with systemic ketoconazole, cyclosporin, itraconazole, dronedarone or verapamil | |
| Rivaroxaban | Contraindicated in patients with following circumstances: |
| Known hypersensitivity | |
| Creatinine clearance <30 mL/min for therapeutic dose or CrCl <15 mL/min for prophylactic dose [prevention of VTE after elective total hip replacement (THR) or total knee replacement (TKR)] | |
| Clinically significant active bleeding | |
| Significant inherited or acquired bleeding disorder | |
| Hepatic disease with coagulopathy (Child-Pugh B and C) | |
| Organ lesions at risk of bleeding including intracranial haemorrhage in previous six months | |
| Indwelling spinal or epidural catheter and during the first six hours after removal | |
| Mechanical heart valve | |
| Pregnancy or breastfeeding mother | |
| Concomitantly treated with strong inhibitors of both CYP 3A4 and P-glycoprotein such as HIV protease inhibitors or systemically administered azole antimycotics | |
| Apixaban | Contraindicated in patients with following circumstances: |
| Known hypersensitivity | |
| Creatinine clearance <25 mL/min | |
| Clinically significant active bleeding | |
| Significant inherited or acquired bleeding disorder | |
| Hepatic disease with coagulopathy (Child-Pugh C) | |
| Organ lesions at risk of bleeding including intracranial haemorrhage in previous 6 months concomitantly treated with strong inhibitors of both CYP 3A4 and P-glycoprotein such as HIV protease inhibitors or systemically administered azole antimycotics | |
| Indwelling spinal or epidural catheter and during the first six hours after removal | |
| Mechanical heart valve | |
| Pregnancy or breastfeeding mother |
Table modified from (
Another limitation in the script-based data analysis is that parental agents are primarily used within hospitals, and thus very few scripts would be written for community use. This is relevant since DOAC use may in part be replacing heparin use, in particular LWMHs such as enoxaparin.
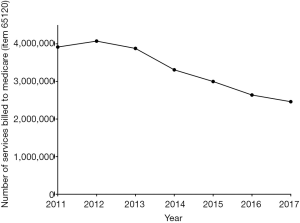
Also relevant to workers in the field and healthcare practice are the changing costs in anticoagulant practice and the potential effect of changing anticoagulant usage on pathology test practice, including laboratory monitoring of anticoagulant therapy. For the latter, laboratory monitoring of VKA therapy, for example, is a key activity of pathology laboratories, and the PT/INR is the most commonly performed coagulation test, and within haematology, perhaps second only to the full blood count. The APTT is the other more commonly performed coagulation test, in some occasions performed for heparin therapy monitoring. Evidence that test numbers are decreasing for performance PT/INR and the APTT, as derived from Medicare data, and for a similar data period of 2011–2017, is shown in Figure 4. This decreasing trend in test requests in Australia is consistent with a reduction in VKA use and associated anticoagulant monitoring (6).
This drop in laboratory PT/INR/APTT testing is not countered by a replacement growth in laboratory DOAC testing, with such requests currently totaling <200/year in our facility, and as compared to nearly 500 requests for LMWH anti-Xa testing and over 70,000 PT/INRs/APTTs (Figure 5). DOAC measuring tests, unlike PT/INR/APTT, do not currently attract Medicare funding in Australia, and thus there is no available national data on test numbers from government sites to our knowledge.

A final consideration is whether the increased usage of DOACs is otherwise affecting haematology laboratory practice, and indeed this is the case. Although, as an example, we are performing a total of <200 tests per year to ‘measure’ drug levels of DOACs, patients on DOACs had well over 2,000 routine coagulation tests performed during the same period in an audit of recent activities (6). This is over 20× the rate of specific DOAC measuring assays. At least some of the routine tests performed on these patients will raise clinical concern with ‘unexpectedly raised’ test times. Further tests (e.g., factor assays) may also be initiated in response, to help explain ‘abnormal’ test findings. Also, many thrombophilia related requests are inappropriately performed on patients on DOACs, and these may cause a range of problems, including misidentification of congenital defects or false identification of lupus anticoagulant (5,17-22).
Conclusions
The use of DOACs continues to grow, both in Australia and worldwide, partly as replacement for VKA therapy, partly as replacement for LMWH therapy, and partly for potentially ‘new’ (previously non-anticoagulated or extended secondary VTE prophylaxis) patients. This trend is expected to continue, in part also considering increases of life expectancy, and since increasing age elevates the risk of many prothrombotic conditions (i.e., VTE, AF). Laboratory test practice is changing in response to these events, with reduced test numbers for PT/INR/APTT, the requirement to measure DOACs on occasion, and the generation of additional haemostasis tests and further complications to laboratory practice associated with DOAC use and effect on a variety of assays. If DOAC influence of test results for other hemostasis assays is not recognised, then this can lead to high potential for disease misdiagnosis. Hematology laboratories worldwide need to be aware of these issues to enable proactive management of changes to testing services. We suspect the situation in Australia is not unique, and we look forward to laboratories in other geographical to share their experience within this issue of the journal.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Blood for the series “Anticoagulant and antithrombotic therapy: globally applied according to local geographical selection criteria”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2018.12.02). The series “Anticoagulant and antithrombotic therapy: globally applied according to local geographical selection criteria” was commissioned by the editorial office without any funding or sponsorship. EJF served as an unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclaimer: The opinions expressed in this review are those of the authors, and not necessarily those of NSW Health Pathology.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Favaloro EJ. Anticoagulant therapy: present and future. Semin Thromb Hemost 2015;41:109-12. [Crossref] [PubMed]
- Favaloro EJ, Lippi G, Koutts J. Laboratory testing of anticoagulants - the present and the future. Pathology 2011;43:682-92. [Crossref] [PubMed]
- Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report. Chest 2018;154:1121-201. [Crossref] [PubMed]
- Baluwala I, Favaloro EJ, Pasalic L. Therapeutic monitoring of unfractionated heparin - trials and tribulations. Expert Rev Hematol 2017;10:595-605. [Crossref] [PubMed]
- Favaloro EJ, Pasalic L, Curnow J, et al. Laboratory monitoring or measurement of direct oral anticoagulants (DOACs): Advantages, limitations and future challenges. Curr Drug Metab 2017;18:598-608. [Crossref] [PubMed]
- Favaloro EJ, Pasalic L, Lippi G. Replacing warfarin therapy with the newer direct oral anticoagulants, or simply a growth in anticoagulation therapy? Implications for Pathology testing. Pathology 2017;49:639-43. [Crossref] [PubMed]
- Favaloro EJ, McCaughan G, Pasalic L. Clinical and laboratory diagnosis of heparin induced thrombocytopenia: An update. Pathology 2017;49:346-55. [Crossref] [PubMed]
- Favaloro EJ, McCaughan G, Mohammed S, et al. HIT or miss? A comprehensive contemporary investigation of laboratory tests for heparin induced thrombocytopenia. Pathology 2018;50:426-36. [Crossref] [PubMed]
- Favaloro EJ. Optimizing the Verification of Mean Normal Prothrombin Time (MNPT) and International Sensitivity Index (ISI) for Accurate Conversion of Prothrombin Time (PT) to International Normalized Ratio (INR). Methods Mol Biol 2017;1646:59-74. [Crossref] [PubMed]
- Bonar R, Favaloro EJ. Explaining and reducing the variation in inter-laboratory reported values for International Normalised Ratio. Thromb Res 2017;150:22-9. [Crossref] [PubMed]
- Favaloro EJ, McVicker W, Mohammed S, et al. Mathematical rounding as a post-analytical issue in pathology reporting: generation of bias in INR resulting. Pathology 2018;50:459-61. [Crossref] [PubMed]
- Jun M, Lix LM, Durand MCanadian Network for Observational Drug Effect Studies (CNODES) Investigators, et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population based, observational study. BMJ 2017;359:j4323. [Crossref] [PubMed]
- Lim HY, Nandurkar H, Ho P. Direct Oral Anticoagulants and the Paradigm Shift in the Management of Venous Thromboembolism. Semin Thromb Hemost 2018;44:261-6. [Crossref] [PubMed]
- Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Emerg Med 2016;34:3-8. [Crossref] [PubMed]
- Iapichino GE, Bianchi P, Ranucci M, et al. Point-of-Care Coagulation Tests Monitoring of Direct Oral Anticoagulants and Their Reversal Therapy: State of the Art. Semin Thromb Hemost 2017;43:423-32. [Crossref] [PubMed]
- Brennan Y, Favaloro EJ, Pasalic L, et al. Lessons learnt from local real-life experience with idarucizumab for the reversal of dabigatran. Intern Med J 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Favaloro EJ, Mohammed S, Curnow J, et al. Laboratory testing for lupus anticoagulant (LA) in patients taking direct oral anticoagulants (DOACs): potential for false positives and false negatives. Pathology 2018; In Press.
- Favaloro EJ. Danger of false negative (exclusion) or false positive (diagnosis) for ‘congenital thrombophilia’ in the age of anticoagulants. Clin Chem Lab Med 2018; [Epub ahead of print]. [Crossref]
- Bonar R, Favaloro EJ, Mohammed S, et al. The effect of dabigatran on haemostasis tests: a comprehensive assessment using in-vitro and ex-vivo samples. Pathology 2015;47:355-64. [Crossref] [PubMed]
- Bonar R, Favaloro EJ, Mohammed S, et al. The effect of the direct factor Xa inhibitors apixaban and rivaroxaban on haemostasis tests: a comprehensive assessment using in vitro and ex vivo samples. Pathology 2016;48:60-71. [Crossref] [PubMed]
- Favaloro EJ, Lippi G. Laboratory testing in the era of direct or non-vitamin k antagonist oral anticoagulants: a practical guide to measuring their activity and avoiding diagnostic errors. Semin Thromb Hemost 2015;41:208-27. [Crossref] [PubMed]
- Gosselin RC, Adcock DM, Bates SM, et al. International Council for Standardization in Haematology (ICSH) Recommendations for Laboratory Measurement of Direct Oral Anticoagulants. Thromb Haemost 2018;118:437-50. [Crossref] [PubMed]
Cite this article as: Favaloro EJ, McCaughan GJB, Mohammed S, Pasalic L. Anticoagulation therapy in Australia. Ann Blood 2018;3:48.


