
Clinical implication of occult hepatitis B infection
Introduction
Occult hepatitis B infection (OBI) is a clinically important but apparently mysterious entity because of the different origination of the patients and different forms of liver involvement. For the former, it depends on the medical history from the patients at the time of diagnosis of the OBI. Generally, patients can be classified into three main groups. First, patients have a definite past history of acute hepatitis B virus (HBV) infection. Second, patients have known chronic hepatitis B (CHB) infection (persistently positive hepatitis B surface antigen (HBsAg) >6 months) and have gone into the last phase of infection i.e., loss of HBsAg (HBsAg seroclearance). Third, patients have no prior medical history of HBV infection and presented at the first time. Among these three possible groups of patients, their share in OBI patient population is yet unknown as there are no large epidemiological studies to address this aspect. It is however anticipated that the second group i.e., CHB infection with HBsAg seroclearance is likely to be the largest group among the pool of patients with OBI, particularly in areas where CHB is of high prevalence. It is suggested that patients with HBV HBsAg gene mutations may also be classified under OBI. The reason is that with the gene mutations, it may alter the HBsAg epitopes which can escape from the detection by commercial HBsAg assays (1-3). However, as shown in several studies, in most of the OBI patients, the HBV does not have the corresponding escape mutations (4-6).
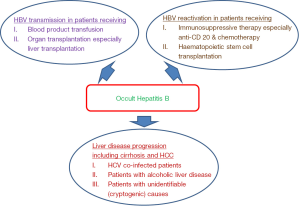
Concerning the clinical significance of OBI, there are several important clinical areas where OBI would play as essential roles (Figure 1). First, it is important to define the transmissibility of HBV infection from OBI subjects. This includes HBV transmission through blood products and organ transplantations from donors with OBI. Second, we also need to understand the effect of OBI on the measurable parameters for liver disease, for example, the liver function, the liver histologic features, and long-term complications including liver cirrhosis and hepatocellular carcinoma (HCC). Third, it is also important to understand whether the presence of OBI in patients with other chronic hepatitis diseases e.g., chronic hepatitis C infection and alcoholic liver disease would have additive or synergistic harmful effects on the development of HCC. Finally, we need to define the risk of HBV reactivation from OBI patients who would receive immunosuppressive therapy.
Patient groups
Patients with acute hepatitis B infection
It has been well documented that the chance of HBV chronicity depends on the age of the subjects when they first contract the HBV. The chances of HBV chronicity are 90%, 30% and 2% for those whose age are <1 years, 1–5 years and >5 years old (7). As such, more than 95% of adults who contract the HBV would not have resulted in HBV chronicity. The majority of these subjects have no symptoms and they develop anti-HBs and anti-HBc during and upon resolution of the acute infection. For those with symptoms of hepatitis e.g., malaise, loss of appetite, nausea and/or tea colour urine, they can provide a more definite history of acute HBV infection. The consequence of “acute” hepatitis B is not as simple as previously believed. In 1994, Michalak and his colleagues documented the presence of HBV DNA in the serum and peripheral blood mononuclear cells (PBMC) in four patients who had recovered up to 70 months after the episodes of the acute HBV infection (8). The persistence of the HBV in subjects with self-limited acute hepatitis B is also confirmed by subsequent studies (9,10). Although there are evidences of viral persistence after acute HBV infection, studies have consistently shown that the necroinflammation in the liver is usually mild (11,12). This finding is also supported by animal experiment in which woodchucks recovered from acute hepatitis have persistently low level of viral replication with mild liver inflammation (13). In addition, all of these subjects have normal liver biochemistry.
It is generally believed that OBI in subjects with acute hepatitis B is innocuous on its own because of the absence of sinister and unfavourable liver parameters. The more important issues arising from these OBI subjects may be the chance of transmission of HBV to uninfected subjects and reactivation of HBV when these OBI subjects received immunosuppressive therapy which will be discussed below.
CHB patients with HBsAg seroclearance
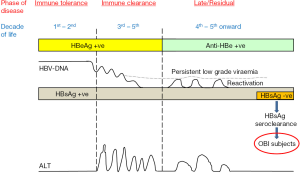
There are classically three phases of CHB infection, namely, immunotolerance phase, immunoclearance phase and residual phase (Figure 2). These phases are distinctly classified according to the viral replication status, immune responses from the host and the resulting serology detected in the patients. HBsAg is consistently positive for the first two phases and most of the patients in the residual phase. However, with the annual incidence between 0.1–0.8% (14), some patients in the residual phase would have HBsAg seroclearance. HBsAg becomes undetectable with decreasing chance of detectable HBV DNA in the serum with the time after HBsAg seroclearance (15). According to Yuen et al., the HBV DNA detectable rates were 13%, 6% and 3.7% after 1, 5–10 and >10 years after HBsAg seroclearance. However, all patients would still have the detectable HBV DNA in the livers (15,16). The viral transcriptional activity is low (nearly absence of mRNA expression for the core, surface, and X genes of the HBV) (15,17). As a result, these patients have minimal or nearly normal liver histology (16,18). According to a study conducted in Hong Kong, of the 29 patients with liver biopsies, 65% had no evidence of necroinflammation and fibrosis. The liver biopsies of the remaining 35% of patients only showed mild necroinflammation and/ or fibrosis (15). In another study conducted in same center, none of the 14 patients with HBsAg seroclearance occurring before age of 40 years had fibrosis in the liver histology (18). Concerning the chance of development of cirrhosis and HCC, one Taiwan study showed that the risk was low if patients had not developed cirrhosis or were not co-infected with other viral hepatitis e.g., hepatitis C (19). Another Taiwan study showed that the cumulative probability of developing these complications was 30% after 4 years of HBsAg seroclearance (20). Further studies in Hong Kong showed that there is no decrease in the risk of development of HCC if the patients had established cirrhosis before HBsAg seroclearance or had HBsAg seroclearance after the age of 50 years (15,18). The 10-year cumulative incidences of development of HCC for patients with HBsAg seroclearance after the age of 50 years and before the age of 50 years are 10% and 0% (P=0.004) respectively (15). It should also be noted that approximately 10% of patients receiving long-term anti-viral treatment would achieve HBsAg seroclearance (21). Whether the favourable clinical outcome observed in patients with early spontaneous HBsAg seroclearance is also found in those with drug-induced HBsAg seroclearance requires further investigations.
Patients with no prior history of hepatitis B infection
Patients with OBI are often diagnosed incidentally through the surveillance by blood donation centres which are usually adopting nucleic acid testing (NAT) for blood product screening. As such, these subjects usually have no personal record/history of contracting HBV. They may be belonging to patients with clinically silent acute hepatitis B or patients without known CHB but undergoing HBsAg seroclearance. However, they may also be primary OBI subjects in whom there is no circulating HBsAg all along.
According to a study with 40 blood donors with OBI, 39 (97.5%) and 36 (90%) were positive for anti-HBc and anti-HBs respectively (22). None was negative for both anti-HBc and anti-HBs, suggesting that seronegative OBI is very uncommon. In addition, none was negative for the common surface gene escape mutation e.g., G145R indicating escape from detection by standard commercial HBsAg assay is also uncommon. Of these 40 OBI subjects, all had normal liver biochemistry. The median necroinflammation and fibrosis scores were 1 and 0, respectively. None had Ishak fibrosis score ≥2. Intrahepatic total HBV DNA, cccDNA, and pregenomic RNA were detectable in 30 (77%), 1 (3%) and 5 (13%) OBI subjects, respectively. The median intrahepatic total HBV DNA (0.22 copies/cell) was low. Eighteen (45%) OBI subjects had quantifiable serum HBV DNA (>1.1 IU/mL).
Specific clinical aspects of OBI
HBV transmission
There are two more common settings whereby HBV transmission is of greater concern. They are recipients receiving blood products coming from OBI donors and recipients receiving organ transplantation, in particular liver transplantation from OBI donors.
Blood donation products containing HBV DNA are potentially infectious and hence transmissible. Animal studies using chimpanzees showed that the minimum 50% infectious dose of HBV was as low as 10 copies of HBV DNA (23). According to a study using chimeric mice (severely immunodeficient) inoculated with sera from OBI donors (24), one of four inoculated mice resulted in having circulating HBV DNA, intrahepatic HBV DNA, covalently closed circular (ccc)DNA and positive HBcAg staining in the liver.
Studies documenting the transmissibility of HBV from OBI subjects are difficult to perform because of several obvious reasons. Most of the studies have to be relied on retrospective data collection and donor-recipient tracing. In addition, the pre-transfusion HBV serology of the recipients is often unknown. These are extremely important information to order to establish the phylogenetic relatedness between donors and recipients. In spite of these difficulties, several studies have proven that the risk is actually quite low (1–3%) (24-26). According to a previous retrospective study, blood components from OBI subjects who were anti-HBs positive are non-infectious (26). According to a study conducted by Japan investigators, of the 12 cases with transfusion-related hepatitis B, all were coming from donors who were negative for both anti-HBc and anti-HBs (presumably during the window phase of the acute HBV infection) (27).
It has been shown that anti-HBc positive blood products from OBI donors are able to cause acute HBV infection in recipients (28-30). Nevertheless, the transmission rate is only 2.2% according to a phylogenetic relatedness study with 95% OBI donors who were positive for anti-HBc (24). The general transmissible rate is estimated to be 2.4–3.0% (14). It seems that although HBV is transmissible through blood products from OBI subjects, the rate of transmission is low. The chance of HBV transmission is dependent on several well-defined parameters, which include the anti-HBs status of both donors and recipients (the presence of anti-HBs in the blood is associated with a significantly lower risk of transmission), the type and the volume of blood products received by the recipients, and the immunocompromised status of the recipients (26,27,31). In addition, as HBV viraemia may fluctuate at low levels at different times, or with different periods of detectable or undetectable HBV DNA within a donor (32), this would also affect the chance of HBV transmission.
Another feasible channel of HBV transmission from OBI donor is in the area of organ transplantation, in particular liver transplantation. It has been shown that de novo HBV infection occurs in 22–100% of recipients receiving liver transplantation from donors who are positive for anti-HBc (33,34). Immunosuppressed recipients who are negative for both anti-HBs and anti-HBc have the highest risk of HBV infection from OBI donors. HBV transmission from sero-negative OBI (negative for both anti-HBc and anti-HBs) remains unknown. In a different scenario, reactivation of HBV in the OBI recipients is possible after liver transplantation because of the immune-compromised status rendered by the immunosuppressive therapy. The natural course of the de novo HBV infection or HBV infection due to HBV reactivation is often less severe than that in recurrent HBV in HBsAg-positive recipients (33).
Unlike in the setting of liver transplantation, the risk of HBV transmission in other organ transplantations e.g., kidney, heart and bone marrow is low (35,36).
To minimize the risk of HBV transmission, anti-viral prophylaxis is always prescribed to the recipients who are going to receive bone marrow or solid organ donations from OBI subjects. Furthermore, even in case of positive anti-HBc donors with undetectable HBV DNA, many centers advocate the use of nucleos(t)ide analogs for recipients.
OBI and chronic liver disease
It has been demonstrated in early 1980s that HBV DNA was detectable in HBsAg-negative patients with chronic liver disease (37,38). From a meta-analysis study, the odds ratio for chronic liver disease from OBI was 8.9 (39). There are different ways to assess the clinical pathological role of OBI. They are studies examining the liver biochemical parameters and the liver histological activities. In general, most of the OBI patients would have normal liver biochemistry with minimal or no necroinflammation and fibrosis in liver histology (22,40-42). However, OBI may still have a considerable risk of the development of liver cirrhosis and HCC. In patients with cryptogenic cause for the liver cirrhosis, it has also been found that 4.8–40% of these patients had OBI (6,43-45).
The role of OBI in chronic liver disease was first widely studied in patients with chronic hepatitis C virus (HCV) infection. There are many studies documenting OBI as one of the etiological factors for the development of cirrhosis and HCC in patients with co-infection with chronic HCV infection (37,46-52). It has been shown that the fibrosis stage is more severe in chronic HCV patients with OBI compared to those without OBI (53). According to a study with 66 chronic HCV patients with OBI, 33% had liver cirrhosis compared to only 19% in chronic HCV patients without OBI (P=0.04) (46). Concerning the risk of HCC in chronic HCV patients with OBI, there are several studies demonstrating that anti-HBc positivity (HR 1.6–3) and HBV DNA positivity are independent risk factors for development of HCC in these patients (51,53,54). These suggest that OBI may be associated with a more progressive course of liver disease in patients with chronic HCV infection. However, several other studies did not observe an increase in disease severity, disease progression and risk of development of HCC in chronic HCV patients with OBI compared with those without OBI (55-59). Nevertheless, a meta-analysis including 12 studies showed that OBI increased the risk of HCC in chronic HCV patients with the HR of 2.8 (52). Taken the results from all studies, it seems that OBI may increase the chance of disease progression and development of HCC in chronic HCV patients. However, it may need more large scale prospective studies to confirm this notion.
Two seminal studies conducted in 1980s had documented the presence of HBV DNA in the liver of HBsAg-negative patients with HCC (60,61). Several other case cohort studies subsequently showed that 45–80% of patients with cryptogenic HCC were harboring the HBV in the liver. The HBV DNA is detectable by PCR assays targeting different genomic regions of the HBV (6,62). The yield is relatively higher in non-tumorous tissues than tumourous counterparts and in X gene than other genes (63). Studies have also shown that the HBV DNA in the liver existed in episomal or integrated forms (6,62). Clonal integration is commonly found in the HCC (64). According to two studies, 48% and 73% patients with cryptogenic HCC had OBI as the only identifiable cause (63,65). Another study had demonstrated that OBI was an independent factor with a relative risk of 8.25 for liver carcinogenesis (54). These studies suggest that OBI is associated with increased risk of HCC (6,63,65,66). This association has been confirmed by a longitudinal follow-up study conducted in Japan (67). Pooling of 16 studies into a meta-analysis, OBI was found to have an increase the risk of development of HCC. The adjusted odds ratio was 2.9 from 5 prospective studies (52). Another meta-analysis including 14 studies also showed the increase risk of HCC in OBI subjects with the risk of 8.9 folds higher than non-infected subjects (39).
There are several possible mechanistic hypotheses explaining the oncogenic property of OBI. First, OBI may have persistent low-grade inflammation leading to or continuing with existing cirrhosis (11,12). Second, the HBV genome of OBI has the oncogenic potential with its possible integration into the human genome as well as with its free episome (6,60,61,68-71). Third, OBI may still have HBV transcriptional activities with viral protein synthesis (e.g., X protein and truncated preS-S protein) which have the transforming properties (72).
HBV reactivation from OBI
HBV reactivation has been well described in HBsAg positive patients receiving immunosuppressive therapy. This entity is now being increasingly recognized in OBI subjects undergoing immunosuppressive therapy (73). The identification of HBV reactivation is clinically very important as fatal hepatic decompensation may develop if the condition is not treated promptly. According to two studies with 468 and 131 HBsAg-negative and anti-HBc positive patients receiving anti-tumour necrosis factor, HBV reactivation rates were found to be 1.7% and 0% respectively (74,75). In another study with 80 HBsAg-negative patients, no HBV reactivation was observed in 34 anti-HBc-negative patients receiving chemotherapy (76). Among the 46 patients with positive anti-HBc, 5 of the 21 (23.8%) patients receiving cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) plus rituximab had HBV reactivation and with one mortality. No HBV reactivation occurred in the remaining 25 anti-HBc positive patients receiving CHOP without rituximab. A prospective study revealed that 41.5% of HBsAg-negative and anti-HBc-positive patients receiving rituximab would develop HBV reactivation (77). Anti-HBs-positive subjects had a significantly lower rate of HBV reactivation (34.4%) compared with anti-HBs-negative subjects (68.3%, P=0.012). Potent B cell depleting agents e.g., rituximab is associated with a very high risk of HBV reactivation in OBI subjects.
Another group of OBI patients who also bear a considerable risk of HBV reactivation are those receiving haematopoietic stem cell transplantation (HSCT) because of prolonged immunosuppressive state. According to different studies, the HBV reactivation rate in HBsAg-negative, anti-HBc positive HSCT recipients ranges from 2.7–42.9% (78-80). From a recent prospective study, the 2-year cumulative HBV reactivation rate was 35% (81). Patients older than 50 years (HR 7.9) and patients with chronic graft-versus-host disease (GVHD) (HR 7.6) are independent factors for HBV reactivation.
There are several possible mechanisms leading to HBV reactivation in OBI subjects undergoing immunosuppressive therapy. First, immunosuppressive agents or chemotherapeutic agents may directly enhance the HBV replication, in particular those with steroid containing regimens as the HBV genome contains glucocorticoid responsive element. Second, through the suppression of the B and T cells function, the immune control on the viral replication is lessened. Third, upon withdrawal immunosuppressive therapy, the rebound of immune activity may exert profound cell mediated damage to the highly HBV loaded hepatocytes.
At present routine prophylactic antiviral agents are recommended for HBsAg positive patients undergoing immunosuppressive therapy. It is generally recommended to start prophylactic anti-viral treatment in HBsAg-negative with or without positive anti-HBc/anti-HBs patients with detectable HBV DNA who are going to receive immunosuppressive therapy/HSCT. For those with undetectable HBV DNA at baseline, regular monitoring at the interval of 1–3 months till at least 12 months after the cessation of immunosuppressive therapy should be implemented. Evidence for the interval of monitoring is lacking. However, the frequency should be dependent on the type of agents being used (for example patients on rituximab should be checked more frequently). The earliest marker for HBV reactivation is the HBV DNA. Recently, two novel emerging HBV serologic tests namely, HBsAg measured by highly sensitive one-step chemiluminescence enzyme immunoassay coupled with an immune complex transfer technique (ICT-CLEIA) (Sysmex corporation, Kobe, Japan) and hepatitis B core-related antigen (HBcrAg) measured by CLEIA using lumipulse, G1200 automated analyzer (Fujirebio Inc., Tokyo, Japan) are being studied for their predictive roles for HBV reactivation in OBI (82,83). They both showed a high predictive value. Until more data are available, it is now recommended that antiviral treatment should be administrated once HBV DNA becomes detectable and should not wait till alanine aminotransferase (ALT) elevation (84). Nucleos(t)ide analogues are very effective in controlling the HBV reactivation with excellent outcome. In a clinical setting where regular HBV DNA monitoring is not feasible, it has also been suggested that patients should be treat pre-emptively (85).
Due to the lack of prospective studies, there are still plenty of potential research areas to define the protocol and treatment regime for HBV reactivation in OBI subjects undergoing immunosuppressive therapy. In the recent prospective studies adopting 4 weekly HBV DNA monitoring in HBsAg-negative, anti-HBc positive patients receiving rituximab or HSCT, with the prompt entecavir treatment once the HBV DNA is detectable, all of the patients achieved excellent control without hepatitis flares (77,81). However, the cost-effectiveness issue incurred with different regimens has not been studied.
Acknowledgments
Funding: MF Yuen received research funding from Bristol-Myers Squibb, Novartis and Gilead Sciences.
Footnote
Conflicts of Interest: MFY received speaker fee from GlaxoSmithKline, Bristol-Myers Squibb, Novartis and Gilead Sciences; and received research funding and is an advisory board member of Bristol-Myers Squibb, Novartis and Gilead Sciences. MFY serves as an unpaid editorial board member of Annals of Blood from Dec 2016 to Dec 2019.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yamamoto K, Horikita M, Tsuda F, et al. Naturally occurring escape mutants of hepatitis B virus with various mutations in the S gene in carriers seropositive for antibody to hepatitis B surface antigen. J Virol 1994;68:2671-6. [PubMed]
- Hou J, Karayiannis P, Waters J, et al. A unique insertion in the S gene of surface antigen--negative hepatitis B virus Chinese carriers. Hepatology 1995;21:273-8. [PubMed]
- Carman WF, Van Deursen FJ, Mimms LT, et al. The prevalence of surface antigen variants of hepatitis B virus in Papua New Guinea, South Africa, and Sardinia. Hepatology 1997;26:1658-6. [Crossref] [PubMed]
- Pollicino T, Raffa G, Costantino L, et al. Molecular and functional analysis of occult hepatitis B virus isolates from patients with hepatocellular carcinoma. Hepatology 2007;45:277-85. [Crossref] [PubMed]
- Huang FY, Wong DK, Seto WK, et al. Sequence variations of full-length hepatitis B virus genomes in Chinese patients with HBsAg-negative hepatitis B infection. PLoS One 2014;9:e99028 [Crossref] [PubMed]
- Pollicino T, Squadrito G, Cerenzia G, et al. Hepatitis B virus maintains its pro-oncogenic properties in the case of occult HBV infection. Gastroenterology 2004;126:102-10. [Crossref] [PubMed]
- Lai CL, Ratziu V, Yuen MF, et al. Viral hepatitis B. Lancet 2003;362:2089-94. [Crossref] [PubMed]
- Michalak TI, Pasquinelli C, Guilhot S, et al. Hepatitis B virus persistence after recovery from acute viral hepatitis. J Clin Invest 1994;93:230-9. [Crossref] [PubMed]
- Yotsuyanagi H, Yasuda K, Iino S, et al. Persistent viremia after recovery from self-limited acute hepatitis B. Hepatology 1998;27:1377-82. [Crossref] [PubMed]
- Penna A, Artini M, Cavalli A, et al. Long-lasting memory T cell responses following self-limited acute hepatitis B. J Clin Invest 1996;98:1185-94. [Crossref] [PubMed]
- Bläckberg J, Kidd-Ljunggren K. Occult hepatitis B virus after acute self-limited infection persisting for 30 years without sequence variation. J Hepatol 2000;33:992-7. [Crossref] [PubMed]
- Yuki N, Nagaoka T, Yamashiro M, et al. Long-term histologic and virologic outcomes of acute self-limited hepatitis B. Hepatology 2003;37:1172-9. [Crossref] [PubMed]
- Mulrooney-Cousins PM, Michalak TI. Persistent occult hepatitis B virus infection: experimental findings and clinical implications. World J Gastroenterol 2007;13:5682-6. [Crossref] [PubMed]
- Lai CL, Yuen MF. Occult hepatitis B infection: Incidence, detection and clinical implications. ISBT Science Series 2009;4:347-51. [Crossref]
- Yuen MF, Wong DK, Fung J, et al. HBsAg seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology 2008;135:1192-9. [Crossref] [PubMed]
- Ahn SH, Park YN, Park JY, et al. Long-term clinical and histological outcomes in patients with spontaneous hepatitis B surface antigen seroclearance. J Hepatol 2005;42:188-94. [Crossref] [PubMed]
- Kuhns M, McNamara A, Mason A, et al. Serum and liver hepatitis B virus DNA in chronic hepatitis B after sustained loss of surface antigen. Gastroenterology 1992;103:1649-56. [Crossref] [PubMed]
- Yuen MF, Wong DK, Sablon E, et al. HBsAg seroclearance in chronic hepatitis B in the Chinese: virological, histological, and clinical aspects. Hepatology 2004;39:1694-701. [Crossref] [PubMed]
- Chen YC, Sheen IS, Chu CM, et al. Prognosis following spontaneous HBsAg seroclearance in chronic hepatitis B patients with or without concurrent infection. Gastroenterology 2002;123:1084-9. [Crossref] [PubMed]
- Huo TI, Wu JC, Lee PC, et al. Sero-clearance of hepatitis B surface antigen in chronic carriers does not necessarily imply a good prognosis. Hepatology 1998;28:231-6. [Crossref] [PubMed]
- Yuen MF, Ahn SH, Chen DS, et al. Chronic hepatitis B virus infection: Disease revisit and management recommendations. J Clin Gastroenterol 2016;50:286-94. [Crossref] [PubMed]
- Wong DK, Fung J, Lee CK, et al. Intrahepatic hepatitis B virus replication and liver histology in subjects with occult hepatitis B infection. Clin Microbiol Infect 2016;22:290.e1-3. [Crossref] [PubMed]
- Komiya Y, Katayama K, Yugi H, et al. Minimum infectious dose of hepatitis B virus in chimpanzees and difference in the dynamics of viremia between genotype A and genotype C. Transfusion 2008;48:286-94. [PubMed]
- Yuen MF, Wong DK, Lee CK, et al. Transmissibility of hepatitis B virus (HBV) infection through blood transfusion from blood donors with occult HBV infection. Clin Infect Dis 2011;52:624-32. [Crossref] [PubMed]
- Candotti D, Allain JP. Transfusion-transmitted hepatitis B virus infection. J Hepatol 2009;51:798-809. [Crossref] [PubMed]
- Mosley JW, Stevens CE, Aach RD, et al. Donor screening for antibody to hepatitis B core antigen and hepatitis B virus infection in transfusion recipients. Transfusion 1995;35:5-12. [Crossref] [PubMed]
- Satake M, Taira R, Yugi H, et al. Infectivity of blood components with low hepatitis B virus DNA levels identified in a lookback program. Transfusion 2007;47:1197-205. [Crossref] [PubMed]
- Hoofnagle JH, Seeff LB, Bales ZB, et al. Type B hepatitis after transfusion with blood containing antibody to hepatitis B core antigen. N Engl J Med 1978;298:1379-83. [Crossref] [PubMed]
- Lander JJ, Gitnick GL, Gelb LH, et al. Anticore antibody screening of transfused blood. Ox. Vox Sang 1978;34:77-80. [Crossref] [PubMed]
- Koziol DE, Holland PV, Alling DW. Antibody to hepatitis B core antigen as a paradoxical marker for non-A, non-B hepatitis agents in donated blood. Ann Intern Med 1986;104:488-95. [Crossref] [PubMed]
- Raimondo G, Caccamo G, Filomia R, et al. Occult HBV infection. Semin Immunopathol 2013;35:39-52. [Crossref] [PubMed]
- Chemin I, Guillaud O, Queyron PC, et al. Close monitoring of serum HBV DNA levels and liver enzymes levels is most useful in the management of patients with occult HBV infection. J Hepatol 2009;51:824-5. [Crossref] [PubMed]
- Dickson RC, Everhart JE, Lake JR, et al. Transmission of hepatitis B by transplantation of livers from donors positive for antibody to hepatitis B core antigen. The National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Gastroenterology 1997;113:1668-74. [Crossref] [PubMed]
- Muñoz SJ. Use of hepatitis B core antibody-positive donors for liver transplantation. Liver Transpl 2002;8:S82-7. [Crossref] [PubMed]
- Wachs ME, Amend WJ, Ascher NL, et al. The risk of transmission of hepatitis B from HBsAg(-), HBcAb(+), HBIgM(-) organ donors. Transplantation 1995;59:230-4. [Crossref] [PubMed]
- De Feo TM, Poli F, Mozzi F, et al. Risk of transmission of hepatitis B virus from anti-HBC positive cadaveric organ donors: a collaborative study. Transplant Proc 2005;37:1238-9. [Crossref] [PubMed]
- Bréchot C, Degos F, Lugassy C, et al. Hepatitis B virus DNA in patients with chronic liver disease and negative tests for hepatitis B surface antigen. N Engl J Med 1985;312:270-6. [Crossref] [PubMed]
- Brechot C, Hadchouel M, Scotto J, et al. Detection of hepatitis B virus DNA in liver and serum: a direct appraisal of the chronic carrier state. Lancet 1981;2:765-8. [Crossref] [PubMed]
- Covolo L, Pollicino T, Raimondo G, et al. Occult hepatitis B virus and the risk for chronic liver disease: a meta-analysis. Dig Liver Dis 2013;45:238-44. [Crossref] [PubMed]
- Yuen MF, Lee CK, Wong DK, et al. Prevalence of occult hepatitis B infection in a highly endemic area for chronic hepatitis B: a study of a large blood donor population. Gut 2010;59:1389-93. [Crossref] [PubMed]
- Fung J, Lai CL, Chan SC, et al. Correlation of liver stiffness and histological features in healthy persons and in patients with occult hepatitis B, chronic active hepatitis B, or hepatitis B cirrhosis. Am J Gastroenterol 2010;105:1116-22. [Crossref] [PubMed]
- Wong DK, Fung J, Lee CK, et al. Hepatitis B virus serological and virological activities in blood donors with occult hepatitis B. Hepatol Int 2014;8:S149.
- Hou J, Wang Z, Cheng J, et al. Prevalence of naturally occurring surface gene variants of hepatitis B virus in nonimmunized surface antigen-negative Chinese carriers. Hepatology 2001;34:1027-34. [Crossref] [PubMed]
- Bréchot C, Thiers V, Kremsdorf D, et al. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely “occult”? Hepatology 2001;34:194-203. [Crossref] [PubMed]
- Alavian SM, Miri SM, Hollinger FB, et al. Occult Hepatitis B (OBH) in Clinical Settings. Hepat Mon 2012;12:e6126 [Crossref] [PubMed]
- Cacciola I, Pollicino T, Squadrito G, et al. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. N Engl J Med 1999;341:22-6. [Crossref] [PubMed]
- Fukuda R, Ishimura N, Niigaki M, et al. Serologically silent hepatitis B virus coinfection in patients with hepatitis C virus-associated chronic liver disease: clinical and virological significance. J Med Virol 1999;58:201-7. [Crossref] [PubMed]
- Tanaka T, Inoue K, Hayashi Y, et al. Virological significance of low-level hepatitis B virus infection in patients with hepatitis C virus associated liver disease. J Med Virol 2004;72:223-9. [Crossref] [PubMed]
- Squadrito G, Pollicino T, Cacciola I, et al. Occult hepatitis B virus infection is associated with the development of hepatocellular carcinoma in chronic hepatitis C patients. Cancer 2006;106:1326-30. [Crossref] [PubMed]
- Shetty K, Hussain M, Nei L, et al. Prevalence and significance of occult hepatitis B in a liver transplant population with chronic hepatitis C. Liver Transpl 2008;14:534-40. [Crossref] [PubMed]
- Adachi S, Shibuya A, Miura Y, et al. Impact of occult hepatitis B virus infection and prior hepatitis B virus infection on development of hepatocellular carcinoma in patients with liver cirrhosis due to hepatitis C virus. Scand J Gastroenterol 2008;43:849-56. [Crossref] [PubMed]
- Shi Y, Wu YH, Wu W, et al. Association between occult hepatitis B infection and the risk of hepatocellular carcinoma: a meta-analysis. Liver Int 2012;32:231-40. [Crossref] [PubMed]
- Matsuoka S, Nirei K, Tamura A, et al. Influence of occult hepatitis B virus coinfection on the incidence of fibrosis and hepatocellular carcinoma in chronic hepatitis C. Intervirology 2008;51:352-61. [Crossref] [PubMed]
- Ikeda K, Marusawa H, Osaki Y, et al. Antibody to hepatitis B core antigen and risk for hepatitis C-related hepatocellular carcinoma: a prospective study. Ann Intern Med 2007;146:649-56. [Crossref] [PubMed]
- Sagnelli E, Imparato M, Coppola N, et al. Diagnosis and clinical impact of occult hepatitis B infection in patients with biopsy proven chronic hepatitis C: a multicenter study. J Med Virol 2008;80:1547-53. [Crossref] [PubMed]
- Kao JH, Chen PJ, Lai MY, et al. Occult hepatitis B virus infection and clinical outcomes of patients with chronic hepatitis C. J Clin Microbiol 2002;40:4068-71. [Crossref] [PubMed]
- Tsubouchi N, Uto H, Kumagai K, et al. Impact of antibody to hepatitis B core antigen on the clinical course of hepatitis C virus carriers in a hyperendemic area in Japan: A community-based cohort study. Hepatol Res 2013;43:1130-8. [Crossref] [PubMed]
- Chen HY, Su TH, Tseng TC, et al. Impact of occult hepatitis B on the clinical outcomes of patients with chronic hepatitis C virus infection: A 10-year follow-up. J Formos Med Assoc 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Cho J, Lee SS, Choi YS, et al. Occult hepatitis B virus infection is not associated with disease progression of chronic hepatitis C virus infection. World J Gastroenterol 2016;22:9427-36. [Crossref] [PubMed]
- Bréchot C, Hadchouel M, Scotto J, et al. State of hepatitis B virus DNA in hepatocytes of patients with hepatitis B surface antigen-positive and -negative liver diseases. Proc Natl Acad Sci U S A 1981;78:3906-10. [Crossref] [PubMed]
- Shafritz DA, Shouval D, Sherman HI, et al. Integration of hepatitis B virus DNA into the genome of liver cells in chronic liver disease and hepatocellular carcinoma. Studies in percutaneous liver biopsies and post-mortem tissue specimens. N Engl J Med 1981;305:1067-73. [Crossref] [PubMed]
- Paterlini P, Gerken G, Nakajima E, et al. Polymerase chain reaction to detect hepatitis B virus DNA and RNA sequences in primary liver cancers from patients negative for hepatitis B surface antigen. N Engl J Med 1990;323:80-5. [Crossref] [PubMed]
- Wong DK, Huang FY, Lai CL, et al. Occult hepatitis B infection and HBV replicative activity in patients with cryptogenic cause of hepatocellular carcinoma. Hepatology 2011;54:829-36. [Crossref] [PubMed]
- Brechot C, Pourcel C, Louise A, et al. Detection of hepatitis B virus DNA sequences in human hepatocellular carcinoma in an integrated form. Prog Med Virol 1981;27:99-102. [PubMed]
- Yotsuyanagi H, Shintani Y, Moriya K, et al. Virologic analysis of non-B, non-C hepatocellular carcinoma in Japan: frequent involvement of hepatitis B virus. J Infect Dis 2000;181:1920-8. [Crossref] [PubMed]
- Fang Y, Shang QL, Liu JY, et al. Prevalence of occult hepatitis B virus infection among hepatopathy patients and healthy people in China. J Infect 2009;58:383-8. [Crossref] [PubMed]
- Ikeda K, Kobayashi M, Someya T, et al. Occult hepatitis B virus infection increases hepatocellular carcinogenesis by eight times in patients with non-B, non-C liver cirrhosis: a cohort study. J Viral Hepat 2009;16:437-43. [Crossref] [PubMed]
- Tamori A, Nishiguchi S, Kubo S, et al. Possible contribution to hepatocarcinogenesis of X transcript of hepatitis B virus in Japanese patients with hepatitis C virus. Hepatology 1999;29:1429-34. [Crossref] [PubMed]
- Paterlini P, Poussin K, Kew M, et al. Selective accumulation of the X transcript of hepatitis B virus in patients negative for hepatitis B surface antigen with hepatocellular carcinoma. Hepatology 1995;21:313-21. [PubMed]
- Bréchot C, Gozuacik D, Murakami Y, et al. Molecular bases for the development of hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC). Semin Cancer Biol 2000;10:211-31. [Crossref] [PubMed]
- Saitta C, Tripodi G, Barbera A, et al. Hepatitis B virus (HBV) DNA integration in patients with occult HBV infection and hepatocellular carcinoma. Liver Int 2015;35:2311-7. [Crossref] [PubMed]
- Cougot D, Neuveut C, Buendia MA. HBV induced carcinogenesis. J Clin Virol 2005;34:S75-8. [Crossref] [PubMed]
- Yuen MF. Need to improve awareness and management of hepatitis B reactivation in patients receiving immunosuppressive therapy. Hepatol Int 2016;10:102-5. [Crossref] [PubMed]
- Lee YH, Bae SC, Song GG. Hepatitis B virus (HBV) reactivation in rheumatic patients with hepatitis core antigen (HBV occult carriers) undergoing anti-tumor necrosis factor therapy. Clin Exp Rheumatol 2013;31:118-21. [PubMed]
- Giannitti C, Lopalco G, Vitale A, et al. Long-term safety of anti-TNF agents on the liver of patients with spondyloarthritis and potential occult hepatitis B viral infection: an observational multicentre study. Clin Exp Rheumatol 2017;35:93-97. [PubMed]
- Yeo W, Chan TC, Leung NW, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol 2009;27:605-11. [Crossref] [PubMed]
- Seto WK, Chan TS, Hwang YY, et al. Hepatitis B reactivation in patients with prior HBV exposure undergoing rituximab-containing chemotherapy for lymphoma: a prospective study. J Clin Oncol 2014;32:3736-43. [Crossref] [PubMed]
- Viganò M, Vener C, Lampertico P, et al. Risk of hepatitis B surface antigen seroreversion after allogeneic hematopoietic SCT. Bone Marrow Transplant 2011;46:125-31. [Crossref] [PubMed]
- Hammond SP, Borchelt AM, Ukomadu C, et al. Hepatitis B virus reactivation following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2009;15:1049-59. [Crossref] [PubMed]
- Nakamoto S, Kanda T, Nakaseko C, et al. Reactivation of hepatitis B virus in hematopoietic stem cell transplant recipients in Japan: efficacy of nucleos(t)ide analogues for prevention and treatment. Int J Mol Sci 2014;15:21455-67. [Crossref] [PubMed]
- Seto WK, Chan TS, Hwang YY, et al. Hepatitis B reactivation in occult viral carriers undergoing hematopoietic stem cell transplantation: a prospective study. Hepatology 2017;65:1451-61. [Crossref] [PubMed]
- Seto WK, Wong DH, Chan TY, et al. Association of hepatitis B core-related antigen with hepatitis B virus reactivation in occult viral carriers undergoing high-risk immunosuppressive therapy. Am J Gastroenterol 2016;111:1788-95. [Crossref] [PubMed]
- Shinkai N, Kusumoto S, Murakami S, et al. Novel monitoring of hepatitis B reactivation based on ultra-high sensitive hepatitis B surface antigen assay. Liver Int 2017; [Epub ahead of print]. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol 2012;57:167-85. [Crossref] [PubMed]
- Marzano A, Angelucci E, Andreone P, et al. Prophylaxis and treatment of hepatitis B in immunocompromised patients. Dig Liver Dis 2007;39:397-408. [Crossref] [PubMed]
Cite this article as: Yuen MF. Clinical implication of occult hepatitis B infection. Ann Blood 2017;2:5.


