
Blinatumomab as frontline therapy for B-cell precursor acute lymphoblastic leukemia in a critically ill young adult: a case report
Highlight box
Key findings
• The use of blinatumomab as targeted immunotherapy showed favorable outcomes in early stages of Philadelphia chromosome-negative (Ph-negative) B-cell precursor acute lymphoblastic leukemia (B-ALL).
What is known and what is new?
• Blinatumomab is a bispecific T-cell engager currently approved for the treatment of relapsed/refractory and minimal residual disease-positive B-ALL. Its utilization as front-line therapy in early stages of the disease is not fully elucidated yet.
• Our case report showcases the use of blinatumomab as first-line therapy in a newly diagnosed critically ill young adult with Ph-negative B-ALL. The treatment showed a safe adverse-effect profile with the achievement of complete remission.
What is the implication, and what should change now?
• Blinatumomab is gaining importance as frontline therapy and may be a new standard of care for B-ALL. It is vitally important to conduct well-designed clinical trials framing the use of blinatumomab in early stages of the disease.
Introduction
Acute lymphoblastic leukemia (ALL) is a malignant proliferation of clonal immature lymphoid cells, which invade the bone marrow (BM), blood, and extramedullary sites (1). Children under 6 years of age are predominantly affected, with a second peak in incidence in adults over 60 years old. It is unknown why Hispanic populations from Central and South America have the highest incidence (2). Many risk factors for the development of this disease in children have been studied, such as genetic syndromes (e.g., Down’s syndrome, Fanconi anemia, etc.) and environmental factors (e.g., ionizing radiation, pesticide exposure). However, most adult patients with ALL do not exhibit any identifiable risk factor (1).
Prognosis depends on age, and clinical, molecular and cytogenetic features. Typically, patients between 1 and 10 years old have the best long-term outcomes, as they usually have low-risk characteristics such as hyperdiploidy and t(Jeny12;21). In contrast, adolescent and young adults (AYA) and adults tend to have high-risk abnormalities such as BCR-ABL1 rearrangement and Philadelphia chromosome-like disease (3-5).
AYA populations with ALL represent a unique complex group, particularly, with respect to the therapeutic approach. Despite the major differences regarding the cytogenetic and molecular features of ALL in AYAs and pediatric patients, the current trend is to treat AYAs with pediatric ALL treatment regimens. Higher survival rates have been obtained when enrolled in trials that applied pediatric-based therapy (6,7).
With greater comprehension of the genetic and molecular basis of ALL, novel drugs have been moving toward frontline and second line therapy. Blinatumomab is a bispecific T-cell engager that has been approved by the Food and Drugs Administration (FDA) for the treatment of relapsed/refractory (r/r) and minimal residual disease (MRD)-positive B-cell precursor ALL (B-ALL) (8). Several clinical trials have obtained favorable outcomes regarding the utilization of blinatumomab in various chemotherapy regimens in B-ALL, especially, during consolidation cycles (9). Nevertheless, the safety and efficacy of blinatumomab in early stages of the disease as frontline therapy is not fully elucidated. This report aims to describe a case of a young adult newly diagnosed with Philadelphia chromosome-negative B-ALL (Ph-negative B-ALL) who received treatment with blinatumomab as induction therapy in combination with chemotherapy in the context of critical illness. We present this case in accordance with the CARE reporting checklist (available at https://aob.amegroups.com/article/view/10.21037/aob-24-7/rc).
Case presentation
A 20-year-old male was referred to our hospital in critical condition for untreated leukemia complicated with sepsis and respiratory failure. Prior to admission to the other hospital, the patient had been suffering from fever, fatigue, malaise, and shortness of breath. He also presented with petechiae and pallor. The patient evolved unfavorably despite the administration of broad-spectrum antibiotics and antifungal coverage requiring intensive care unit (ICU) admission under invasive mechanical ventilation and hemodynamic support with vasopressors. He was then referred to our center, which had a higher resolutive capacity for diagnosis and treatment.
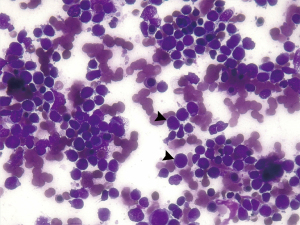
On admission, the white blood cell (WBC) count was 1,370/mm3, the hemoglobin (Hb) was 10.1 g/dL and the platelet count was 42,000/mm3. Serum albumin was measured at 2.80 g/dL. Serum lactic dehydrogenase was 542 U/L, serum fibrinogen was 893 mg/dL, prothrombin time and international normalized ratio were prolonged. Two sets of blood cultures were taken, with a negative reading at 7 days. A BM smear showed a marked proliferation of lymphoid lineage (Figure 1). Flow cytometry showed B-lymphoid blast infiltration of 71.50%, predominantly in Pro-B stage: CD45 +, CD34 −/++, CD19 +/++, CD38 −/+, CD123 −/+, CD66c −/+++, CD10 −/+++, CD22 +/++ and CD81 +/++; negative for CD20, cyIgM, cyCD3, cyMPO, CD7 and smCD3 (Figure 2). The karyotype was complex: 46, XY, del(Jeny9)(p21), der(Jeny17)t(Jeny1;17)(p21;p13)[23]/46, XY[07] (Figure 3). Molecular testing did not detect BCR-ABL1. The patient was diagnosed with Ph-negative B-ALL.


Dexamethasone at 8 mg twice daily was initiated for 4 days with tapering to 4 mg twice daily, alongside with filgrastim 300 mcg once daily for 3 days. Broad-spectrum antibiotics, antifungal coverage and prophylaxis against herpes simplex virus and Pneumocystis jirovecii were given. A lumbar puncture was performed to rule out lymphoblastic infiltration of the central nervous system, and flow cytometry showed no infiltration on cerebrospinal fluid. Prophylactic intrathecal chemotherapy with cytarabine 50 mg, methotrexate 12.5 mg, and dexamethasone 4 mg was administered.
Due to the patient’s critical condition, we initiated targeted immunotherapy with blinatumomab followed by chemotherapy. On day 11 of hospitalization (DH11), blinatumomab 9 µg per day as a continuous infusion was initiated and was administered for seven days. Prior to immunotherapy, the WBC count was 2,430/mm3, the Hb was 9 g/dL and the platelet count was 96,000/mm3. C reactive protein (CRP) was 1.18 mg/dL and procalcitonin was 0.18 ng/mL. Shortly after the administration of blinatumomab, the patient presented a febrile episode with no signs or symptoms of neurological involvement. The WBC count dropped to 700/mm3, CRP increased to 2.93 mg/dL, serum ferritin was >2,000 ng/mL and sedimentation velocity was 22 mm/h. Interleukin-6 was measured at 264.8 pg/mL and cytokine release syndrome (CRS) was suspected. Supportive measures were delivered and blinatumomab infusion was maintained with close monitorization of inflammatory markers. The WBC count increased to 2,560/mm3 and the inflammatory markers decreased. On DH18, the dose of blinatumomab was increased to 28 mcg per day. On DH26, a BM aspiration showed complete morphologic remission and flow cytometry showed an MRD of 0.05%. Blinatumomab infusion continued until DH31. Next, the patient received intravenous chemotherapy with vincristine 2 mg on DH32, 39, 46 and 53; daunorubicin 50 mg on DH39 and 46; and oral prednisone 20 mg twice daily. On DH53, BM aspiration showed complete morphologic remission, and flow cytometry of BM showed MRD negativity by the end of chemo-immunotherapy induction. The patient then underwent consolidation treatment with chemotherapy under the HOVON 146 ALL trial protocol (10) and, currently, is under evaluation for haploidentical allograft. The complete timeline for the diagnostic tests and therapeutic agents used is illustrated in Tables 1,2.
Table 1
| Therapy and diagnostic tests | Day of hospitalization | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DH1 | DH2 | DH3 | DH4 | DH5 | DH6 | DH7 | DH8 | DH9 | DH10 | |
| Broad-spectrum antibiotics | X | X | X | X | X | X | X | X | X | X |
| Antifungal therapy | X | X | X | X | X | X | X | X | X | X |
| HSV and Pneumocystis jirovecii prophylaxis | X | X | X | X | X | X | X | X | X | X |
| Dexamethasone 8 mg twice daily | X | X | X | X | ||||||
| Dexamethasone 4 mg twice daily | X | X | X | X | X | |||||
| Filgrastim 300 mcg once daily | X | X | X | |||||||
| Bone marrow aspiration with flow cytometry | B-lymphoid blast infiltration of 71.5% | Complex karyotype | ||||||||
| Prophylactic intrathecal chemotherapy | X | |||||||||
| Lumbar punction with flow cytometry | No CSF infiltration | |||||||||
| White blood cell count (per mm3) | 1,370 | 1,130 | 790 | 1,540 | 2,060 | 1,950 | 1,830 | 1,590 | 1,830 | 1,790 |
| Hemoglobin (g/dL) | 10.1 | 9.3 | 8.5 | 10.4 | 10.3 | 9.9 | 9 | 7.7 | 8.9 | 8.9 |
| Platelet count (per mm3) | 42,000 | 44,000 | 45,000 | 55,000 | 51,000 | 48,000 | 50,000 | 43,000 | 111,600 | 98,000 |
| Serum fibrinogen (mg/dL) | 893 | 357 | 252 | |||||||
| INR | 1.5 | 1.3 | 1.4 | 1.4 | 1.3 | 1.5 | ||||
| C reactive protein (mg/dL) | 23.4 | 16.7 | 8.2 | 3.6 | 1.4 | 1.8 | 1 | 0.7 | 0.7 | 0.4 |
| Procalcitonin (ng/mL) | 1.45 | 1.13 | 0.66 | 0.28 | 0.09 | |||||
X, the patient received the drug in this day. DH, day of hospitalization; HSV, herpes simplex virus; CSF, cerebrospinal fluid; INR, international normalized ratio.
Table 2
| Therapy and diagnostic tests | DH11 (DT1) | DH12 (DT2) | DH13 (DT3) | DH14 (DT4) | DH15 (DT5) | DH16 (DT6) | DH17 (DT7) | DH18 (DT8) | DH19 (DT9) | DH20 (DT10) | DH21 (DT11) | DH22 (DT12) | DH23 (DT13) | DH24 (DT14) | DH25 (DT15) | DH26 (DT16) | DH27–DH31 (DT17–DT21) | DH32–DH52 (DT22–DT42) | DH53 (DT43) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Broad-spectrum antibiotics | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Antifungal therapy | X | X | X | X | X | X | X | X | X | X | |||||||||
| HSV and Pneumocystis jirovecii prophylaxis | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Dexamethasone 16 mg once daily | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| Blinatumomab 9 µg—continuous infusion | X | X | X | X | X | X | X | ||||||||||||
| Blinatumomab 28 µg—continuous infusion | X | X | X | X | X | X | X | X | X | X | |||||||||
| Chemotherapy (intravenous vincristine and daunorubicin, and oral prednisone) | X | X | |||||||||||||||||
| Bone marrow aspiration with flow cytometry | MRD of 0.05% | MRD (−) | |||||||||||||||||
| White blood cell count (per mm3) | 2,430 | 700 | 940 | 2,560 | 1,850 | 1,690 | 1,580 | 1,810 | 2,480 | 2,500 | 2,960 | ||||||||
| Hemoglobin (g/dL) | 9 | 10.3 | 9.8 | 9.2 | 9.5 | 8.6 | 8.5 | 8.7 | 8.2 | 8.4 | 10 | ||||||||
| Platelet count (per mm3) | 96,000 | 98,000 | 100,000 | 93,000 | 103,000 | 102,000 | 115,000 | 140,000 | 149,000 | 140,000 | 143,000 | ||||||||
| Serum fibrinogen (mg/dL) | 294 | 404 | 289 | 210 | 192 | ||||||||||||||
| INR | 1.7 | 1.4 | 1.3 | 1.3 | 1.2 | ||||||||||||||
| C reactive protein (mg/dL) | 1.2 | 2.9 | 8.9 | 5.5 | 2.4 | 0.7 | 0.2 | 0.2 | 0.2 | 0.1 | |||||||||
| Sedimentation velocity (mm/h) | 23 | 22 | 49 | 29 | 21 | ||||||||||||||
| Procalcitonin (ng/mL) | 0.18 | 0.33 | 0.35 | 0.03 | |||||||||||||||
| IL-6 (pg/mL) | 14.3 | 264.8 | 5.5 | 5.8 |
X, the patient received the drug in this day. DH, day of hospitalization; DT, day of immunotherapy of chemotherapy; HSV, herpes simplex virus; MRD, minimal residual disease; INR, international normalized ratio; IL-6, interleukin-6.
All procedures performed in this study were in accordance with the ethical standards of the Institutional Review Board of the British American Hospital (CIEI/CAA-055-2023) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
A 20-year-old male with newly diagnosed Ph-negative B-ALL was treated with blinatumomab in combination with chemotherapy agents as frontline therapy. The patient achieved MRD negativity after receiving blinatumomab in a continuous infusion over 21 days alongside intrathecal chemotherapy with cytarabine, methotrexate and dexamethasone, and then followed by intravenous chemotherapy with vincristine, daunorubicin and prednisone. During the early course of administration of blinatumomab, Grade 1 CRS was suspected. Supportive measures were delivered, and the infusion was continued without any further complications.
Treatment of B-ALL has evolved dramatically in recent years, as the genetic and molecular characteristics of the disease have become better understood (8). AYA populations are a unique and complex group that have been favored by the administration of pediatric-based instead of adult-based therapy regimens. Nevertheless, the outcomes are not as promising as their counterparts, paving the way for novel treatments (11). Blinatumomab is a bispecific monoclonal antibody that binds to CD19 antigen on B-lymphoblasts and CD3 on T cells. It is currently approved by the FDA for the treatment of r/r and MRD-positive B-ALL (8). It has shown longer overall survival (OS) rate and higher rate of disease-free survival (DFS) in r/r B-ALL as compared with standard-of-care chemotherapy (12,13). Blinatumomab also demonstrated favorable outcomes in MRD-positive B-ALL (14).
Considering its promising activity as targeted immunotherapy and its relatively safe adverse-effect profile, blinatumomab is advancing toward a frontline position in the treatment of B-ALL. The rationale for incorporating blinatumomab in early stages of the disease is to achieve greater outcomes in comparison to late stages, where it is less likely to be curable (15). The use of blinatumomab as a chemotherapy-sparing first line agent, specially in chemotherapy-intolerant or chemotherapy-resistant B-ALL, is a novel approach currently under investigation. Blinatumomab exhibits a safer adverse-effect profile compared to conventional chemotherapy, with lower rates of myelosuppression and mucosal toxicity (16). It also has proven to be effective as a first-line therapy in chemotherapy-intolerant patients (17). The early incorporation of blinatumomab reduces the burden associated with chemotherapy, specially in critically ill patient unsuitable for intensive regimens, while attaining favorable outcomes. However, it has been called into question in which extent chemotherapy-sparing alternatives may be incorporated as frontline therapy without diminishing short- and long-term outcomes (15).
Several clinical trials support the administration of blinatumomab during consolidation cycles (9). The GIMEMA LAL2317 showed the efficacy of sequential administration of blinatumomab during early and late consolidation in increasing the MRD conversion rate, OS and DFS (18). Moreover, phase 3 ECOG-1910 trial showed longer OS and lower deaths from ALL in the blinatumomab arm (19). Clinical trials have also addressed the efficacy of blinatumomab administration during early stages of the disease for the purpose of reducing chemotherapy burden. The ALLG ALL8 study addressed blinatumomab administration following debulking chemotherapy with promising results (20). Moreover, blinatumomab has also proved to be efficient in the treatment of high-risk ALL with poor prognostic factors. The administration of blinatumomab during consolidation and maintenance in patients with KMT2A rearrangements, IKZF1 intragenic deletion or MRD-positive following induction was proved to be efficient (21). Additionally, utilization of blinatumomab as prephase therapy in patients who received pediatric B-ALL protocols proved to be safe and achieved greater complete morphologic remission and MRD negativity rates (22).
Conclusions
Blinatumomab, as targeted immunotherapy, has shown promising outcomes when administered in early stages of the disease. Furthermore, blinatumomab exhibits a safe adverse-effect profile that has positioned itself as a chemotherapy-sparing agent, which is particularly important in critically ill patients unsuitable for intensive chemotherapy. Hence, it is gaining importance as frontline therapy and may be a new standard of care for B-ALL. Nevertheless, certain studies showed preliminary results in limited follow-up periods. Therefore, it is vitally important to conduct well-designed clinical trials framing the use of blinatumomab in early stages of the disease. Most of the studies assessed the administration of blinatumomab during consolidation cycles. Thus, it would be interesting to address blinatumomab in newly diagnosed patients as an induction therapy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://aob.amegroups.com/article/view/10.21037/aob-24-7/rc
Peer Review File: Available at https://aob.amegroups.com/article/view/10.21037/aob-24-7/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-24-7/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the Institutional Review Board of the British American Hospital (CIEI/CAA-055-2023) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Malard F, Mohty M. Acute lymphoblastic leukaemia. Lancet 2020;395:1146-62. [Crossref] [PubMed]
- Laurini JA, Perry AM, Boilesen E, et al. Classification of non-Hodgkin lymphoma in Central and South America: a review of 1028 cases. Blood 2012;120:4795-801. [Crossref] [PubMed]
- Rubnitz JE, Pui CH, Downing JR. The role of TEL fusion genes in pediatric leukemias. Leukemia 1999;13:6-13. [Crossref] [PubMed]
- Schafer ES, Hunger SP. Optimal therapy for acute lymphoblastic leukemia in adolescents and young adults. Nat Rev Clin Oncol 2011;8:417-24. [Crossref] [PubMed]
- Malouf C, Ottersbach K. Molecular processes involved in B cell acute lymphoblastic leukaemia. Cell Mol Life Sci 2018;75:417-46. [Crossref] [PubMed]
- Derwich K, Brzezinski A, Karpenko C, et al. Acute Lymphoblastic Leukemia in Adolescents and Young Adults: A Polish Perspective. J Adolesc Young Adult Oncol 2022;11:1-5. [Crossref] [PubMed]
- Rytting ME, Jabbour EJ, O'Brien SM, et al. Acute lymphoblastic leukemia in adolescents and young adults. Cancer 2017;123:2398-403. [Crossref] [PubMed]
- FDA. Drug Approvals and Databases. Available online: https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases
- Pourhassan H, Agrawal V, Pullarkat V, et al. Positioning blinatumomab in the frontline of adult B-cell acute lymphoblastic leukemia treatment. Front Oncol 2023;13:1237031. [Crossref] [PubMed]
- HOVON. HOVON HO146 ALL. Available online: https://hovon.nl/nl/trials/ho146
- Hanbali A, Kotb A, Fakih RE, et al. Improved survival in adolescents and young adults (AYA) patients aged 14-55 years with acute lymphoblastic leukemia using pediatric-inspired protocol - a retrospective analysis of a real-world experience in 79 of patients treated at a national tertiary care referral center. Leuk Res Rep 2021;16:100270. [Crossref] [PubMed]
- Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N Engl J Med 2017;376:836-47. [Crossref] [PubMed]
- Drawdy L, Jones LA, Hall PD. Blinatumomab: A Step Forward in the Treatment of B-Cell Precursor Acute Lymphoblastic Leukemia. J Hematol Oncol Pharm 2019;9:38-46.
- Jabbour EJ, Short NJ, Jain N, et al. Blinatumomab is associated with favorable outcomes in patients with B-cell lineage acute lymphoblastic leukemia and positive measurable residual disease at a threshold of 10-4 and higher. Am J Hematol 2022;97:1135-41. [Crossref] [PubMed]
- Franquiz MJ, Short NJ. Blinatumomab for the Treatment of Adult B-Cell Acute Lymphoblastic Leukemia: Toward a New Era of Targeted Immunotherapy. Biologics 2020;14:23-34. [PubMed]
- So W, Pandya S, Quilitz R, et al. Infectious Risks and Complications in Adult Leukemic Patients Receiving Blinatumomab. Mediterr J Hematol Infect Dis 2018;10:e2018029. [Crossref] [PubMed]
- Hodder A, Mishra AK, Enshaei A, et al. Blinatumomab for First-Line Treatment of Children and Young Persons With B-ALL. J Clin Oncol 2024;42:907-14. [Crossref] [PubMed]
- Bassan R, Chiaretti S, Della I, et al. Preliminary results of the GIMEMA LAL2317 sequential chemotherapy-blinatumomab front-line trial for newly diagnosed adult Ph-negative B-lineage all patients. European Hematology Association - EHA 2021. Available online: https://library.ehaweb.org/eha/2021/eha2021-virtual-congress/324522/renato.bassan.preliminary.results.of.the.gimema.lal2317.sequential.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Asearch%3Ds114
- Litzow MR, Sun Z, Paietta E, et al. Consolidation Therapy with Blinatumomab Improves Overall Survival in Newly Diagnosed Adult Patients with B-Lineage Acute Lymphoblastic Leukemia in Measurable Residual Disease Negative Remission: Results from the ECOG-ACRIN E1910 Randomized Phase III National Cooperative Clinical Trials Network Trial. Blood 2022;140:LBA-1. [Crossref]
- Fleming S, Reynolds J, Bajel A, et al. P365: Sequential blinatumomab with reduced intensity chemotherapy for older adults with newly diagnosed Ph- B-Precursor acute lymphoblastic leukemia – final results of the ALLG ALL08 study. Hemasphere 2023;7:e811479d. [Crossref]
- Boissel N, Huguet F, Graux C, et al. Frontline Consolidation with Blinatumomab for High-Risk Philadelphia-Negative Acute Lymphoblastic Adult Patients. Early Results from the Graall-2014-QUEST Phase 2. Blood 2021;138:1232. [Crossref]
- Rijneveld A, Gradowska P, Bellido M, et al. P366: Blinatumomab added to prephase and consolidation therapy in newly diagnosed precursor B-ALL in adults. a phase II Hovon Trial. Hemasphere 2022;6:266-7. [Crossref]
Cite this article as: Mendez-Guerra C, Reyes-Farias CI, Uribe-Ramirez L, Carrasco-Yalan A. Blinatumomab as frontline therapy for B-cell precursor acute lymphoblastic leukemia in a critically ill young adult: a case report. Ann Blood 2024;9:18.


