
Intravenous immunoglobulin can induce antibody-dependent cell-mediated cytotoxicity and upregulate FCGR3A and FcγRIIIA expression in vitro
Highlight box
Key findings
• Intravenous immunoglobulin (IVIG) increases FCGR3A expression in effector cells with a corresponding increase in FcγRIIIA expression on the surface of effector cells, indicating that IVIG plays a role in regulating the Fc fragment receptor on the surface of effector cells.
What is known and what is new?
• Activating FcγRI is a high-affinity receptor, and FcγRIIB can negatively regulate signals.
• The IVIG Fc fragment-binding activity to FcγRIIIA and evaluation of the IVIG-mediated antibody-dependent cell-mediated cytotoxicity (ADCC).
What is the implication, and what should change now?
• FcγRIIIA may be a key mediator of the immunomodulatory effects of IVIG, and the findings highlight the potential for further research into the mechanisms underlying the IVIG-mediated ADCC in the treatment of cancer and other diseases.
• FcγRIIIA of IVIG needs more attention.
Introduction
Intravenous immunoglobulin (IVIG) is a biological product derived from a pool with thousands of healthy plasma donors, and contains 95% immunoglobulin G (IgG) with traces of immunoglobulin A (IgA) and immunoglobulin M (IgM) (1,2). Due to its abundant immunoglobulin content, IVIG is widely used in the treatment of patients with various immunodeficiency and inflammatory states (1,3,4). Although IVIG has a long history of clinical application, its mechanism is complex and remains unclear. Notably, it exerts its essential function through the Fc fragment-binding to Fc receptors on immune cells that act as activators and inhibitors (3). The FcγR family comprises several activating receptors and one inhibitory receptor. Activating receptor FcγRI is a high-affinity receptor for IgG that can recognize particular IgG subclasses in their monomeric forms (5). FcγRI mainly expresses on monocytes/macrophages, dendritic cells (DCs), and activated neutrophils. One of the major functions of FcγRI is to activate myeloid cells to phagocytose IgG1 and IgG-bound target cells via antibody dependent cellular phagocytosis (ADCP) (6). Inhibitory receptor FcγRIIB contains a typical immunoreceptor tyrosine inhibitory motif and can negatively regulate signals. When FcγRIIB binds to an immune complex, ITIM becomes phosphorylated and subsequently recruits phosphatases such as Src homologous region tyrosine kinase suppressor protein that inhibit the downstream activation signaling pathway, induce inhibition of B cell receptor or FcγR-mediated signaling, and reduces the proliferation of B cells and antibody production (7,8). These interactions cause inhibitory signals that weaken cell activation.
FcγRIIIA is associated with antibody-dependent cell-mediated cytotoxicity (ADCC), an adaptive immune response (9). Antibodies predominantly exert ADCC through the Fc fragment binding to FcγRIIIA , subsequently driving effector cells to kill the target cells (10). Many cells, including natural killer cells (NK cells) and neutrophils, can act as effector cells and kill target cells through the release of perforin and granzymes (11). Relatively little is known about FcγRIIIA, and its potential mechanism remains to be elucidated.
To better understand the biological functions of IVIG, we investigated the IVIG-mediated ADCC. The key point for detecting ADCC is cell cytotoxicity assay. In this study, a time-resolved fluorescence (TRF) cell cytotoxicity assay was selected as a non-radioactive method, providing safe and specific results. Simultaneously, reporter cells were chosen to detect the IVIG Fc fragment-binding activity to FcγRIIIA. The Jurkat-NFAT-Luc-CD16 cell line was stably transfected with FcγRIIIA receptor and nuclear factor of activated T-cells (12). The luminescence released by the reporter cells reflected the biological activity of ADCC. Meanwhile, activating mRNAs for FCGR3A were detected to investigate the regulation of the IVIG-mediated ADCC. We present this article in accordance with the MDAR reporting checklist (available at https://aob.amegroups.com/article/view/10.21037/aob-23-42/rc).
Methods
Evaluation of the IVIG-mediated ADCC by the TRF assay
IVIG (5%; pH4; Shandong Taibang Biological Products Company, Tai’an, Shandong, China) was diluted to a final concentration of 1.25 mg/mL using culture medium containing 10% fetal bovine serum (FBS) (FuHeng Cell Center, Shanghai, China). K562 cells (FuHeng Cell Center) were harvested from cultures as the target cells, and adjusted to 1×106 cells/mL in loading buffer [phosphate-buffered sale (PBS) containing 10% FBS and 20 mM HEPES]. Next, 3 µL of BATDA (fluorescence-enhancing ligand) reagent (Perkin Elmer, Shanghai, China) was added to 2 mL of cells, mixed gently, and incubated for 15 min at 37 ℃ under 5% CO2 in a cell culture incubator, followed by centrifugation (800 ×g, 5 min) and three washes with wash buffer (PBS containing 20 mM HEPES). The loaded cells were resuspended, adjusted to 2×105 cells/mL for plating in the assay, and preincubated with IVIG for 30 min at 37 ℃ under 5% CO2. Heparinized venous blood was obtained from healthy donors (n=3). After the preincubation, 100 µL of fresh whole blood cells was added, and the mixture was incubated in the cell culture incubator at 37 ℃ under 5% CO2 for 2 h. Fluorescence signals were detected using a compatible plate reader (EnVision®; Perkin Elmer, Waltham, MA, USA) with TRF function (excitation 340 nm and emission at 615 nm). All samples were measured in triplicate.
Evaluation of the IVIG Fc fragment-binding activity to FcγRIIIA
Jurkat-NFAT-Luc-CD16 cells (Vazyme, Nanjing, China) were chosen as the effector cells and PLC/PRF/5 cells (FuHeng Cell Center) expressing hepatitis B surface antigen (HBsAg) on the cell membrane were used as the target cells. Dilute IVIG (100 mg/mL) fivefold with culture medium [Roswell Park Memorial Institute (RPMI) medium containing 10% FBS and 5% penicillin-streptomycin solution] to achieve a starting working concentration of 20 mg/mL, designated as concentration 1, followed by serial two-fold dilutions to obtain concentrations 2, 3, ..., up to concentration 9, yielding a total of nine working concentrations for the samples. After incubation of the effector cells and target cells with IVIG (10%; pH4; Shanxi Kangbao Biological Product Company, Changzhi, Shanxi, China), the Fc fragment-binding activity to FcγRIIIA was observed by detecting luciferase release. All samples were measured in triplicate.
Detection of the FCGR3A expression after IVIG incubation with effector cells
The FCGR3A sequence was searched in the National Center for Biotechnology Information database, and coherent primers were designed. Next, 3 mL of fresh whole blood cells was incubated with of 100 µL IVIG (50 mg/mL) for 2 h, and Trizol (Thermo Fisher, Waltham, MA, USA) was added to extract mRNA. Total RNA was isolated from the samples using TRUEscript RT MasterMix [for real-time polymerase chain reaction (PCR)] (DF Biotech, Chengdu, China) in accordance with the manufacturer’s instructions. An aliquot (2 mg) of total RNA was subjected to first-strand cDNA synthesis using a TransScript First-Strand cDNA Synthesis Kit (AiDLAB Biotech, Beijing, China), and FCGR3A gene expression was determined by quantitative real-time PCR using SYBR Green QPCR Mix (DF Biotech). The housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was amplified using the following primer set: GAPDH-F, 5'-CGGAGTCAACGGATTTGGTC-3'; GAPDH-R, 5'-CGGTGCCATGGAATTTGCCA-3'.
Detection of the FcγRIIIA expression on effector cells
FcγRIIIA (CD16) is a low-affinity receptor for IgG crystallizable fragments on effector cells. To quantify FcγRIIIA externalization during IVIG moderation of effector cells, 100 µL of whole blood cells was extracted from fresh anticoagulated whole blood and incubated with K562 cells and IVIG for 2 h at 37 ℃ under 5% CO2. PE-CD16 (BD Biosciences, Franklin Lakes, NJ, USA) was added and incubated at 4 ℃ for 30 min, before the samples were subjected to flow cytometric analysis in ACSCelesta™ Cell Analyzer (BD Biosciences).
Statistical analysis
PCR measurement data was analyzed by unpaired t-test and FcγRIIIA expression on effector cells were analyzed by one-way analysis of variance, A four-parameter curve equation regression model was applied for the IVIG Fc fragment-binding activity to FcγRIIIA. Values of P<0.05 were considered statistically significant. All analysts and diagrams were performed by GraphPad Prism 8.0.
Ethical consideration
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of the Institute of Blood Transfusion, Chinese Academy of Medical Sciences (IRB approval number: 2022021). Written informed consent was obtained from all participants prior to enrollment.
Results
The IVIG-mediated ADCC by the TRF assay
As shown in Figure 1, at the concentration of 1.25 mg/mL, the IVIG-mediated ADCC reached 40% and killed more than twice the number of K562 cells compared with the control cells. The specific ADCC value for combined whole blood cells and K562 cells was approximately 20% without IVIG, and the activity increased to approximately 40% in the presence of IVIG.
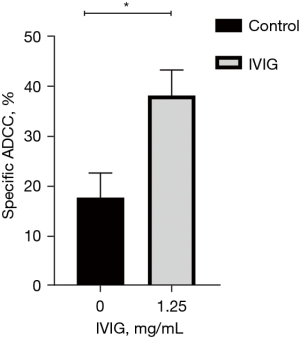
The IVIG Fc fragment-binding activity to FcγRIIIA
The luminescence signals reflected the IVIG Fc fragment-binding activity to FcγRIIIA with activity increasing from 40 to 2,000 luminescence relative units (LRU) as the concentration of IVIG increased (Figure 2A). We created a four-parameter curve equation regression model, and the concentration of IVIG was transformed to log X. A fitted curve was obtained in the four-parameter curve equation regression model, and the dose-effect curve for ADCC showed a typical S-shape with R2=0.99 (Figure 2B).
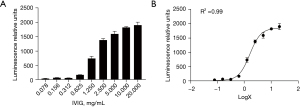
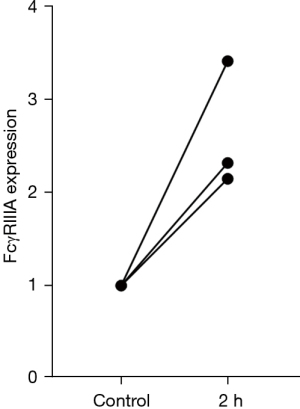
Regulation of FCGR3A synthesis by IVIG
The expression levels of FCGR3A increased after incubation with IVIG (n=3). Synthesis of cDNAs was conducted, and the relative gene expression levels were calculated and plotted in a graph. Similar to the ADCC findings, the expression levels of FCGR3A increased by almost two-fold after the addition of IVIG, and one donor was even detected with three-fold higher expression compared with the control cells (Figure 3).
Regulation of FcγRIIIA expression on effector cells by IVIG
Compared with the control cells, the expression of FcγRIIIA on the surface of whole blood cells incubated with IVIG was upregulated by almost two-fold. When whole blood cells were incubated without IVIG, the CD16 level was 33.7% (Figure 4A). After addition of IVIG, the CD16 level markedly increased to 66.7% (Figure 4B). These findings suggested that direct engagement of FcγRIIIA by IVIG may be responsible for the ADCC. The expression levels of FcγRIIIA increased after incubation with IVIG, consistent with the augmentation of FCGR3A synthesis (Figure 4).

Discussion
As an important blood product, IVIG is widely used in the treatment of patients with approved indications, including primary and secondary immune deficiency diseases, infectious diseases, and respiratory or other system diseases (4). Simultaneously, IVIG also has many off-label applications (4). The Fab fragment has a broad spectrum for recognition of many antigen types, and therefore it is difficult to investigate the mechanism of IVIG through the Fab fragment. Research on the mechanism of action of IVIG has mainly focused on the activation of FcγRI and the inhibitory effect of FcγRIIB (3). Few studies have been conducted on FcγRIIIA. Aloulou and colleagues proposed that the IVIG Fc fragment binds to the Fc receptors on immune cells through FcγRIIIA is important for anti-inflammatory effects and immune regulation (13), and suggested that further investigation on the role of FcγRIIIA in IVIG-mediated immunomodulation was warranted. Whereas the IVIG-mediated ADCC has rarely been described. Specifically, we aimed to determine the potential role of FcγRIIIA in the anti-tumor effects of IVIG. Whole blood contains NK cells, and activated NK cells release perforin, granzyme B, and other substances. Perforin forms “pores” in the cell membrane that allow water and electrolytes to rapidly enter the cell, resulting in cell lysis. Meanwhile, granzyme B enters the cell through “pores” formed by interferon on the cell membrane, and inducing apoptosis of the target cells. IVIG can activate the ADCC effects of NK cells through FcγRIIIA and cause lysis of K562 cells. It should be pointed out that the reason why we chose whole blood cells instead of purified cultured NK cells is that the pH of IVIG (4.0) may affect the activity of cells. The cells in fresh peripheral blood represent the authentic environment in vivo and can provide stable and reliable results. Meanwhile, during in vitro culture, interleukin-2 (IL-2) stimulation is required to maintain the biological activity, and this cannot represent the natural activity of the cells to a certain extent.
As the effector cells, we chose transgenic reporter Jurkat-NFAT-Luc-CD16 cells, which are widely used for the detection of ADCC activity in monoclonal antibodies and are the most stable reporter cells expressing FcγRIIIA identified to date. We did consider using primary cells as the effector cells, but the primary cells from donors were unstable and had different immune backgrounds, and the cell extraction progress was complicated and resulted in different immune responses.
HBsAg was only expressed on the surface of the target cells after binding with the Fab fragment of IVIG, and then the IVIG Fc fragment bond to FcγRIIIA on the surface of Jurkat-NFAT-Luc-CD16 cells. Before the Fc fragment activate FcγRIIIA, the Fab fragment needs to bind to an antigen. However, as mentioned earlier, IVIG is produced from a pool of healthy human plasma samples containing polyclonal antibodies, and it is not possible to select an antigen that is uniquely present in healthy people. Since the selected IVIG was produced in China and the plasma source was the Chinese population, we considered the background of hepatitis B vaccination in the Chinese population and selected HBsAg as the antigen.
Previous studies have not evaluated the IVIG Fc fragment-binding activity to FcγRIIIA. Notably, IVIG showed optimal binding activity to FcγRIIIA. However, compared with the ADCC of monoclonal antibody drugs, where the LRU value can reach tens of thousands or even hundreds of thousands (12), the measured LRU value of IVIG was relatively low. This finding is speculated to be related to the diversity of the IVIG antibodies, because IVIG contains thousands of polyclonal antibodies and its targeting activity toward specific antigens is not as good as that of monoclonal antibodies. At the same time, IVIG contains natural IgG with weaker affinity for effector cell surface receptors than monoclonal antibodies.
It is worth noting that our result for the IVIG-mediated ADCC is controversial with respect to the current limited research data. Dominguez-Soto and colleagues reported that IVIG could inhibit the growth of tumor cells by regulating the polarization of macrophages (14). Damianovich and colleagues conducted in vitro and in vivo experiments to evaluate the effect of IVIG on metastatic ability, and found that IVIG could inhibit the proliferation and invasion of tumor cells in mice (15). IVIG showed a significant inhibitory effect on tumor cells in vivo. However, Pradier and colleagues demonstrated that IVIG could inhibit the activity of NK cells, thereby inhibiting the biological activity of ADCC (16). We reached a different conclusion in our experiments using whole blood cells. Unlike the previous measurement methods for cytotoxicity, we did not use peripheral blood mononuclear cells or pure NK cells as the effector cells. The low pH of IVIG (4.0) may affect the activity of cells, with a phenol red indicator changing from red to white. Meanwhile, isolated pure NK cells require IL-2 stimulation to maintain the biological effect (16), whereas fresh whole blood cells can provide a more stable and powerful buffering effect, representing more realistic and reliable results for an in vitro environment. However, the reaction process in the system could not be directly observed, and we have not yet found a way of combining IVIG and K562 cells, which we will continue to explore in future studies.
Regarding agglutinin, the Pharmacopoeia of the People’s Republic of China (17) indicates that the agglutinin in IVIG should be lower than 1:64, and thus agglutinin should not affect the experiments. At the same time, the index we observed was the lysis of K562 cells, which may not be affected by lectins. We did not take the blood types of the donors into account during the collection as long as the donors met the criteria for blood donation. The donor information was recorded and we can explore the influence of blood types on the IVIG-mediated ADCC in the future.
Our results showed that IVIG could increase FCGR3A expression in the effector cells, with corresponding increases in FCGR3A on the surface of the effector cells, indicating that IVIG has a role in regulating the Fc fragment receptor on the surface of the effector cells. Jacobi and colleagues found that IVIG could reduce the level of FcγRIIIA on the surface of effector cells (18), which is different from our conclusion. The reasons for the difference may be as follows. First of all, it could be related to the incubation time of IVIG and effector cells. In our experimental design, the incubation time of IVIG and effector cells was 24 h, Whereas, Jacobi and colleagues used 3 h. A longer incubation time can better reflect the changes regulated by IVIG. Furthermore, it could be related to the working concentration of IVIG. Our working concentration of IVIG was 11.5 mg/mL, while Jacobi and colleagues used 20 mg/mL. Human plasma already contains IgG, and thus extra IgG in IVIG may block the Fc receptor on the surface of the effector cells and inhibit the activity. In addition, the receptors expressed on the surface of effector cells are heterogeneous, and therefore IVIG may bind to different receptors and exert different regulatory effects, resulting in different experimental results. Compared with Jacobi and colleagues, we not only detected the expression of FcγRIIIA on the surface of effector cells, but also detected the expression of FCGR3A. At the same time, we added Fc fragment blockers before detecting FcγRIIIA on the surface of effector cells, thereby preventing the non-specific binding activity of IVIG and effector cells from causing false-negative results. Our results suggest that IVIG can regulate the expression of FcγRIIIA on effector cells, which may be one of the mechanisms through which IVIG exerts the biological effects of ADCC.
The IVIG Fc fragment-binding activity to FcγRIIIA warrants more attention to promote applications of IVIG. In future studies, we will examine the activation of the receptor signaling pathway after immune cell binding to IVIG and the resulting effects, to further clarify the mechanism of action of IVIG.
Conclusions
This study investigated the IVIG-mediated ADCC, including measurement of the IVIG Fc fragment-binding activity to FcγRIIIA and the regulation of FcγRIIIA expression by IVIG. The results indicate that ADCC that can kill tumor cells and IVIG can also promote FCGR3A synthesis, increasing FcγRIIIA expression on immune cells membranes. The findings provide further evidence for the IVIG-mediated ADCC and understanding of the biological activity of IVIG.
Acknowledgments
The authors thank Alison Sherwin, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn/) for editing the English text of a draft of this article.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://aob.amegroups.com/article/view/10.21037/aob-23-42/rc
Data Sharing Statement: Available at https://aob.amegroups.com/article/view/10.21037/aob-23-42/dss
Peer Review File: Available at https://aob.amegroups.com/article/view/10.21037/aob-23-42/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-23-42/coif). The authors reported that this study was supported by the Medical and Health Science and Technology Innovation Project, Chinese Academy of Medical Sciences (grant Nos. 2021-12M-I-060 and 2021-12M-1-042), the NMPA Key Laboratory of Quality Control of Blood Products (supported by the Guangdong Institute for Drug Control) (grant No. KF2021011), and the Sichuan Science and Technology Program (grant No. 2022YFS0010). The authors also reported receipt of part of the materials and drugs including IVIG from G.Y. G.Y. serves as an employee of Zhengzhou RAAS Blood Products Co., Ltd. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of the Institute of Blood Transfusion, Chinese Academy of Medical Sciences (IRB approval number: 2022021). Written informed consent was obtained from all participants prior to enrollment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol 2017;29:491-8. [Crossref] [PubMed]
- Yamamoto T, Cui Y, Patel D, et al. Effect of intravenous immunoglobulin (IVIg) on primate complement-dependent cytotoxicity of genetically engineered pig cells: relevance to clinical xenotransplantation. Sci Rep 2020;10:11747. [Crossref] [PubMed]
- Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol 2013;13:176-89. [Crossref] [PubMed]
- Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: A review of evidence. J Allergy Clin Immunol 2017;139:S1-S46. [Crossref] [PubMed]
- Nagelkerke SQ, Dekkers G, Kustiawan I, et al. Inhibition of FcγR-mediated phagocytosis by IVIg is independent of IgG-Fc sialylation and FcγRIIb in human macrophages. Blood 2014;124:3709-18. [Crossref] [PubMed]
- Chen X, Song X, Li K, et al. FcγR-Binding Is an Important Functional Attribute for Immune Checkpoint Antibodies in Cancer Immunotherapy. Front Immunol 2019;10:292. [Crossref] [PubMed]
- Pricop L, Redecha P, Teillaud JL, et al. Differential modulation of stimulatory and inhibitory Fc gamma receptors on human monocytes by Th1 and Th2 cytokines. J Immunol 2001;166:531-7. [Crossref] [PubMed]
- Galvez-Cancino F, Simpson AP, Costoya C, et al. Fcγ receptors and immunomodulatory antibodies in cancer. Nat Rev Cancer 2024;24:51-71. [Crossref] [PubMed]
- Sun Y, Izadi S, Callahan M, et al. Antibody-receptor interactions mediate antibody-dependent cellular cytotoxicity. J Biol Chem 2021;297:100826. [Crossref] [PubMed]
- Iannello A, Ahmad A. Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev 2005;24:487-99. [Crossref] [PubMed]
- González-González E, Camacho-Sandoval R, Jiménez-Uribe A, et al. Validation of an ADCC assay using human primary natural killer cells to evaluate biotherapeutic products bearing an Fc region. J Immunol Methods 2019;464:87-94. [Crossref] [PubMed]
- Hsieh YT, Aggarwal P, Cirelli D, et al. Characterization of FcγRIIIA effector cells used in in vitro ADCC bioassay: Comparison of primary NK cells with engineered NK-92 and Jurkat T cells. J Immunol Methods 2017;441:56-66. [Crossref] [PubMed]
- Aloulou M, Ben Mkaddem S, Biarnes-Pelicot M, et al. IgG1 and IVIg induce inhibitory ITAM signaling through FcγRIII controlling inflammatory responses. Blood 2012;119:3084-96. [Crossref] [PubMed]
- Domínguez-Soto A, de las Casas-Engel M, Bragado R, et al. Intravenous immunoglobulin promotes antitumor responses by modulating macrophage polarization. J Immunol 2014;193:5181-9. [Crossref] [PubMed]
- Damianovich M, Solomon AS, Blank M, et al. Attenuation of colon carcinoma tumor spread by intravenous immunoglobulin. Ann N Y Acad Sci 2007;1110:567-77. [Crossref] [PubMed]
- Pradier A, Papaserafeim M, Li N, et al. Small-Molecule Immunosuppressive Drugs and Therapeutic Immunoglobulins Differentially Inhibit NK Cell Effector Functions in vitro. Front Immunol 2019;10:556. [Crossref] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China. Beijing: China Medical Science Press; 2020.
- Jacobi C, Claus M, Wildemann B, et al. Exposure of NK cells to intravenous immunoglobulin induces IFN gamma release and degranulation but inhibits their cytotoxic activity. Clin Immunol 2009;133:393-401. [Crossref] [PubMed]
Cite this article as: Zhu L, Zhao Y, Ye G, Huang Y, Liu Q, Jiang P, Li C, Du X, Ye S, Ma L. Intravenous immunoglobulin can induce antibody-dependent cell-mediated cytotoxicity and upregulate FCGR3A and FcγRIIIA expression in vitro. Ann Blood 2024;9:13.


