Immune-mediated thrombotic thrombocytopenic purpura landscaping in Gulf countries: a real-world evidence study (ATHENA Study)
Highlight box
Key findings
• Caplacizumab shortens platelet normalization time and enhances survival rates.
What is known and what is new?
• This disease greatly burdens patients and healthcare systems in the Gulf region.
• A new management plan for this disease is needed to improve the patient’s health and save healthcare resources.
What is the implication, and what should change now?
• The availability of diagnostic tests is critical for better treatment outcomes in immune-mediated thrombotic thrombocytopenic purpura.
Introduction
Immune-mediated thrombotic thrombocytopenic purpura (iTTP) is a rare, life-threatening disorder resulting in the formation of blood clots in small blood vessels, leading to their occlusion and consequent blood flow restriction to vital organs, including the heart, brain, and kidney (1). These microvascular occlusions lead to the development of microangiopathic hemolytic anemia (MAHA) and multi-organ ischemia (2). TTP is characterized by key symptoms such as a severe degree of thrombocytopenia, MAHA, fever, acute kidney injury, and severe neurologic manifestations (collectively referred to as the pentad) (3). The exact etiology of TTP is still unknown, but the condition is usually associated with reduced or absent activity of the ADAMTS13 enzyme, which is responsible for cleaving von Willebrand factor (VWF) during the clotting process (4). Two forms of TTP are known: immune-mediated [iTTP; also known as acquired TTP (aTTP)] or congenital. The immune-mediated form of TTP is much more common than the congenital form and manifests as antibodies against the ADAMTS13 enzyme (5,6).
Patients with TTP have a mortality rate of up to 90% if left untreated (7). The introduction of therapeutic plasma exchange (TPE) has revolutionized TTP treatment and has increased the probability of 10-year survival to 70–85% (7,8). However, many TTP patients experience refractoriness to different medical treatment options (9), and precipitating factors such as pregnancy, infections, and surgery may lead to a disease relapse episode for up to 30% of survivors (10). There is a high level of discrepancy in the incidence rate of iTTP among different geographical regions due to variability in demographic factors. For instance, annual incidence in Europe ranges from one and a half to six cases/million/year (11-13), while in the United States (US), the incidence is around three cases/million/year (14,15). The incidence rate in the US may be linked to its ethnic makeup, as African Americans have an increased incidence of iTTP compared to other populations (14,16). Unfortunately, data related to TTP epidemiology in the Arabian Gulf region are very scarce, so the local disease burden and spread are not fully understood yet.
A diagnosis of TTP can be reached through a combination of clinical suspicion and laboratory findings. As per recent International Society on Thrombosis and Haemostasis (ISTH) diagnostic guidelines, patients with high clinical suspicion of immune-mediated TTP should provide plasma samples for ADAMTS13 testing before they receive TPE or transfusion of blood products (17). ISTH guidance recommends starting TPE, corticosteroids, and caplacizumab before the results of ADAMTS13 testing become available, but the decision as to whether or not a patient continues caplacizumab should depend on the reported ADAMTS13 level (17), caplacizumab should be continued if the ADAMTS13 activity is <10 IU/dL. A diagnosis of TTP cannot rely on the classic TTP pentad alone, as this only manifests in 50% of cases (18). Patients with TTP must have anemia, thrombocytopenia, and evidence of active hemolysis in their laboratory investigations; laboratory test results that confirm hemolysis include increased reticulocyte count, schistocytes, increased unconjugated bilirubin, elevated lactate dehydrogenase (LDH), and reduced haptoglobin (19,20).
TPE and high-dose steroids are the cornerstones for treating patients with TTP. For the acute management of TTP, patients may require TPE, glucocorticoids, immunosuppressive agents such as rituximab, and anti-VWF agents such as caplacizumab (21). Unfortunately, these drugs and other management approaches are costly and impose a large burden on healthcare resource utilization (HRU). Relatively few clinical trials are conducted in countries located in the Middle East and North Africa (MENA) region, especially for rare disorders such as iTTP.
Therefore, this retrospective study is designed in order to better understand the nature of this disease and the local burden on the patients and healthcare systems in Arabian Gulf countries, with the goal of generating recent and sufficient data for future decision-making and strategic planning. We present this article in accordance with the STROBE reporting checklist (available at https://aob.amegroups.com/article/view/10.21037/aob-23-29/rc).
Methods
Study design, procedures, and objectives
This was a multinational, multicenter, observational, retrospective, noncomparative study that used existing data from electronic and/or paper medical records. A retrospective design was selected for this study to enable the collection of real-world data based on routine care, void of the Hawthorne effect (i.e., changes in behavior based on awareness of being observed) (22). Detailed methodology for this study has previously been described and published (23). Data was captured from different centers/clinics in five Arabian Gulf countries: Kingdom of Saudi Arabia (KSA), United Arab Emirates (UAE), Kuwait, Qatar, and Oman. The primary objective was to explore and identify the nature of iTTP and its local burden (clinical and HRU) in the Arabian Gulf. The secondary objective was to understand the routine management pattern employed for patients with iTTP in this region.
Study population and included sites
Eligible patients were aged ≥12 years, with a clinical diagnosis of iTTP (initial or recurrent) in the past 36 months before the index date (index date: date of medical chart review). Exclusion criteria were as follows: platelet count ≥100×109/L, other known causes of thrombocytopenia [including but not limited to clinical evidence of enteric infection with E. coli 0157 or related organisms; atypical hemolytic uremic syndrome (HUS); hematopoietic stem cell, bone marrow, or organ transplantation associated with thrombotic microangiopathy (TMA); known/suspected sepsis; or disseminated intravascular coagulation (DIC)], or congenital TTP.
Where feasible, sites were selected based on geographic region and institution size in order to capture variations in current real-world patterns of care. In addition, a structured feasibility questionnaire was administered to each potential site/data source as part of the formal qualification process and to facilitate the identification of the most suitable sites for participation in this study before site selection. The feasibility questionnaire was used to collect various types of information, including evaluation of the number of potentially eligible patients, data availability, staff resources, and any unforeseen opportunities and/or challenges.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the institutional review board of participated countries. In Saudi Arabia, IRB approvals were obtained from Jeddah Health Affairs under the IRB KACST, KSA (No. H-02-J-002, on 12/04/2021) and from King Saud University under the IRB KACST (Registration No.: H-01-R-002, on 23/08/2021). In Oman, IRB approval was obtained from the Ministry of Health (MOH/CSR/21/24592, on 04/08/2021). In UAE, approval was obtained from Tawam Hospital (DOH/CVDC/2021/654 SKMC, on 20/05/2021). In Qatar, this study was approved by the National Centre for Cancer Care and Research (Protocol No. MRC-02-21-725). In Kuwait, the study was approved by the Ministry of Health (No.: 1709 / 2020). Individual consent for this retrospective analysis was waived.
Data collection and validation
For all patients, socio-demographic data, nature of iTTP (i.e., new, exacerbation, or relapse), time to platelet normalization, iTTP-related death, co-medications and duration of other treatments, referral scheme, diagnostic tests, laboratory assessments of organ damage markers before and after treatment (including LDH, cardiac troponin, and creatinine level), and healthcare utilization [intensive care unit (ICU) days, hospitalization days, and the number of TPE sessions] were collected. This study had two main retrospective data sources: paper medical records and electronic medical records/electronic health records. A clinical response to treatment is marked by a significant platelet count increase exceeding 150×109/L for two consecutive days, improved hemolysis markers levels (LDH), and evident clinical improvement. Clinical remission is achieved when the platelet count remains standard for the first 30 days post-TPE cessation. Exacerbation is defined as a reduction in platelet count to below the lower limit of the established reference range (e.g., <150×109/L) and elevated LDH levels within those 30 days post-TPE. A relapse denotes the recurrence of an acute TTP episode after initially attaining clinical remission.
The study centers were granted access to electronic case report forms (eCRFs) filled by the investigator or the authorized designee. Computerized handling of the data by the contract research organization RAY (i.e., RAY-CRO) generated additional requests to which the participating investigator was requested to respond by confirming or modifying the data questioned. In addition to automatic validation, a manual/medical review of data generated further queries raised in the system. The site staff were responsible for resolving automatic and manual queries by confirming or modifying the extracted data.
Sample size and statistical considerations
With a prevalence of around 3.7 iTTP cases per million people yearly (24), the proposed average sample size would be 50 patients. However, due to variations in data collection across different countries, a margin of error of 20% (±10 patients) was factored in when determining the initial sample size. Therefore, for this study, a total of 40 to 60 patients were selected for analysis. All statistical analyses were performed at the 5% significance level using two-sided tests or two-sided confidence intervals (CIs). Paired comparisons were performed by paired t-test in case of normally distributed data, and Wilcoxon signed rank-sum test for non-normally distributed data. In non-paired comparisons, an independent t-test was used for normally distributed data, while the Mann-Whitney U test was used for non-normally distributed data.
Time-to-event outcomes were assessed using the Kaplan-Meier method and reported as descriptive statistics (e.g., median time to event) with 95% CIs. Predictions were identified using a multivariate logistic regression model and split-sample validation. Data were summarized separately by country and pooled overall across the countries where local country data protection and privacy regulations were permitted. A log-rank test was also performed to evaluate differences in subgroups of interest in the Kaplan-Meier analyses. In addition, subgroup analyses on treatment patterns and overall survival were carried out for patients treated with or without caplacizumab. Finally, time to platelet count normalization (starting from the date of the first treatment with caplacizumab) was further analyzed in the subgroup of patients treated with caplacizumab. All data analyses were performed using IBM SPSS statistics version 22. P values <0.05 were specified as the threshold for statistical significance.
Results
Participants and demographic characteristics
Data was received for 62 patients and was duly screened, with two records subsequently excluded; in one case, the date of diagnosis was 39 months before the index date, and in the other case, platelet count at diagnosis was unavailable. The most represented country was KSA, with 26 patients (43.3%), followed by Oman with 8 patients (13.3%). The mean age [standard deviation (SD)] of the patients was 40.6 (16.5) years, while the mean age at diagnosis was 39.3 (16.5) years. Most of the included patients were Arabs (n=36; 60.0%), and 53.3% were females. Patients’ nationalities were diverse; however, Saudis were the most represented (n=26; 43.3%). At baseline, the laboratory assessment in the study cohort revealed the following values: the median LDH, cardiac troponin, and creatinine levels were 963 IU/L, 0.08 ng/mL, and 98 µmol/L. The socio-demographic characteristics and laboratory data at diagnosis among the included patients are shown in Table 1 and Table S1.
Table 1
| Characteristics | Values |
|---|---|
| Age (years), mean (SD) | |
| Age at index date | 40.6 (16.5) |
| Age at diagnosis | 39.3 (16.5) |
| Gender, n (%) | |
| Male | 28 (46.7) |
| Female | 32 (53.3) |
| Caplacizumab use, n (%) | |
| Patients received caplacizumab | 7 (11.7) |
| Patients did not receive caplacizumab | 53 (88.3) |
| Caplacizumab dose (n=7), n (%) | |
| 10 mg; intravenous | 5 (71.4) |
| 11 mg; intravenous | 1 (14.3) |
| 10 mg; subcutaneous | 1 (14.3) |
| Platelet count at diagnosis (×109/L) (missing =3), mean (SD) | 19.3 (16.9) |
| Patients with schistocytes at diagnosis (missing =5), n (%) | 54 (90.0) |
| Comorbidities at diagnosis† | 94 (156.7) |
| Lab assessment of organ damage markers (at baseline) | |
| Lactate dehydrogenase level (IU/L), median (range) | 963 (1.08–3,542) |
| Cardiac troponin (ng/mL), median (range) | 0.08 (0–4) |
| Creatinine level (µmol/L), median (range) | 98 (0.9–1,768) |
One patient might be labeled with more than 1 type of iTTP. †, every subject may have more than 1 comorbidity. SD, standard deviation; iTTP, immune-mediated thrombotic thrombocytopenic purpura.
Nature of iTTP cases
Most of the included patients (n=50; 83.3%) were new iTTP cases, and 8 (13.3%) were refractory to treatment. The mean platelet count (SD) was 19.3×109/L (16.9×109/L), and schistocytes were found at diagnosis in the majority of cases (n=54; 90.0%). Neurologic manifestations were the most frequently reported (n=37; 61.7%), followed by renal manifestations (n=19; 31.7%) (Table 1). The detailed manifestations of the associated comorbidities are presented in Table S2.
iTTP disease burden
Time to platelet count response
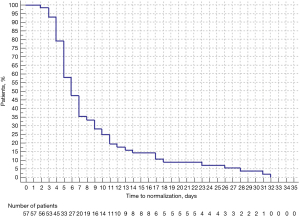
The median [range] time to platelet count normalization was 6 [3–32] days from the date of the first treatment (Table 2 and Figure 1). Notably, this time was shorter for the subgroup of patients who received caplacizumab (n=7) at 4 [2–10] days. In this subgroup, caplacizumab therapy was started a median of 6 [0–16] days after patients experienced their last event (i.e., relapse, refractoriness, or exacerbation). Further analysis showed that patients with measured ADAMTS13 activity had a mean time of 9.47 (±7.06) days for platelet normalization, compared to those without measured ADAMTS13 activity who had a mean time of 9.80 (±8.15) days, with no significant difference observed (P=0.87).
Table 2
| Platelet count response | Time (days) |
|---|---|
| All patients (missing =3) | |
| Median [range] | 6 [3–32] |
| Mean (SD) | 8.8 (7.2) |
| Patients who received caplacizumab (n=7) | |
| Median [range] | 4 [2–10] |
| Mean (SD) | 5.1 (2.7) |
| Duration between the last event† and the caplacizumab start date | |
| Median [range] | 6 [0–16] |
| IQR | 12 |
| Mean (SD) | 6 (5.6) |
The percentage of patients who achieved platelet normalization in more than 6 days was 47.37% (27/57). The percentage of patients who achieved platelet normalization in more than 10 days was 22.81% (13/57). †, including the new cases. SD, standard deviation; IQR, interquartile range.
Assessment of organ damage
The mean difference for cardiac troponin was 0.51 (SD: 0.98) ng/mL. For LDH and creatinine, a statistically significant difference was found between the baseline and after-treatment values (P<0.05) (Table 3). The median [range] time to reach organ damage marker normalization was 8 [0–202] days for LDH, 2.5 [0–9] days for cardiac troponin, and 4 [0–80] days for creatinine.
Table 3
| Organ damage marker | Mean difference | SD | 95% CI | P value |
|---|---|---|---|---|
| Lactate dehydrogenase level (IU/L) | 913.3 | 768.2 | 690.2–1,136.4 | <0.001* |
| Cardiac troponin (ng/mL) | 0.51 | 0.98 | 0–1.174 | 0.118 |
| Creatinine level (µmol/L) | 67.6 | 201.7 | 10.28–124.9 | 0.022* |
*, significant difference. SD, standard deviation; CI, confidence interval.
Survival status
Of the included patients, 6 (10.0%) died during the data collection period, and 4 (6.7%) of these deaths were related to iTTP. The median [range] time to death was 5.5 [1–41] days. There were no deaths reported amongst patients who received caplacizumab.
HRU due to iTTP
Almost all patients were hospitalized (98.3%) during the data collection period. The majority of patients were hospitalized as new cases (81.7%), and 10 patients (16.7%) were hospitalized due to disease recurrence (full details in Table 4). Most of the ICU admissions (n=36) were new iTTP cases (n=35/36); only 1 patient with recurrent disease (exacerbated/relapsed) required ICU admission. TPE was available for both recurrent and new patients (95% in both groups) (Tables S3,S4). The mean length of hospital stay for patients treated with caplacizumab was 18.67 days, while for those who received other regimens, it was 29.46 days (P=0.58), Table S5.
Table 4
| Parameter | Values |
|---|---|
| Hospitalization | |
| Hospitalized patients, n (%) | 59 (98.3) |
| New case (hospitalized), n (%) | 49 (81.7) |
| Exacerbated/relapsed (hospitalized), n (%) | 10 (16.7) |
| Hospitalization days, median [range] | 13 [1–81] |
| ICU admission (missing =4) | |
| Patients admitted to ICU, n (%) | 36 (60.0) |
| ICU days, median [range] | 14.28 [1–72] |
| TPE service | |
| Availability of TPE service, n (%) | 57 (95.0) |
| Number of total TPE sessions taken, median [range] | 8 [1–41] |
iTTP, immune-mediated thrombotic thrombocytopenic purpura; ICU, intensive care unit; TPE, therapeutic plasma exchange.
iTTP management plan
All the study patients received medical treatment during their management journey. The most frequently reported iTTP treatment regimen was TPE + corticosteroids + rituximab (61.7%). All patients who received a regimen of TPE + corticosteroids + rituximab + caplacizumab (10.0%) experienced clinical remission during the study period. We observed a statistically significant difference in clinical remission rates between the studied treatment groups (P=0.04). Detailed data on iTTP management plans are presented in Table 5.
Table 5
| Treatment regimen (n=60) | Number of patients (%) | Clinical remission outcome (%) | P value¶ |
|---|---|---|---|
| TPE + corticosteroids + rituximab + caplacizumab | 6 (10.0) | 6 (100.0) | – |
| TPE + corticosteroids + caplacizumab | 1§ (1.7) | – | 0.04 |
| TPE + corticosteroids† | 16 (26.7) | 9 (56.3) | – |
| TPE + corticosteroids + rituximab‡ | 37 (61.7) | 33 (89.2) | – |
| Total | 60 (100.0) | 48 (80.0) | – |
Every subject may have received more than 1 treatment regimen/drug during the treatment journey. Patient remission was based on the final response to the treatment combination (i.e., the patient might be initially refractory to treatment and then achieve final outcome remission). †, data were not available for 6 patients; ‡, data were not available for 1 patient, data presented as rituximab frequency during the whole study; §, clinical remission data cannot be evaluated for this patient due to their ongoing treatment; ¶, Fisher exact test. iTTP, immune-mediated thrombotic thrombocytopenic purpura; TPE, therapeutic plasma exchange.
Concomitant medications
Over half of the patients (58.3%) used concomitant medications during the study period. The most used medications were insulin (9 patients), omeprazole (8 patients), paracetamol (8 patients), and calcium supplements (6 patients). Full details of concomitant medications are shown in Table S6.
iTTP diagnosis in the Gulf region
Diagnostic tests and grading scores
ADAMTS13 level testing was performed for 33.3% (20 patients) in laboratories outside the country and for 23.3% (14 patients) in the local labs, while 43.3% (26 patients) did not undergo ADAMTS13 testing.
A median [range] of 11 [1–96] days elapsed before test results were received. Also, anti-ADAMTS13 antibody testing was performed in 28 patients (46.7%). Other diagnostic tests performed during treatment included platelet count (n=59; 98.3%), creatinine level (n=57; 95.0%), and bilirubin level (n=55; 91.7%). Details of other diagnostic tests used are shown in Table S7.
Two grading scores, PLASMIC and French TMA, were used for the diagnosis of the disease. The PLASMIC score was used in 36 patients (60.0%), while the French score was used only in 9 patients (15.0%). Regarding the PLASMIC score, most patients scored 6 or 7; for the French TMA score, one patient scored 1, six patients scored 2, and two patients scored 3 (Table 6).
Table 6
| Scoring system | N (%) |
|---|---|
| PLASMIC score (n=36) | |
| Score 2 | 1 (2.8) |
| Score 3 | 1 (2.8) |
| Score 4 | 1 (2.8) |
| Score 5 | 1 (2.8) |
| Score 6 | 16 (44.4) |
| Score 7 | 16 (44.4) |
| French TMAs score (n=9) | |
| Score 1 | 1 (1.7) |
| Score 2 | 6 (10.0) |
| Score 3 | 2 (3.3) |
Missing (n=24) for PLASMIC score. TMAs, thrombotic microangiopathies.
Referral scheme
Most patients (70.5%) were referred to other hospitals during the course of treatment. Reasons for patient referral included unconfirmed diagnoses that needed specific assessment (31.7%), unavailability of treatment resources (11.7%), and unavailability of diagnostic tests (6.7%); 20% of patients were referred due to other causes. Patients spent a median [range] of 3 [1–30] days from the appearance of clinical manifestations to reaching a confirmed diagnosis. Additional details [e.g., name of the referral hospital (if outside the hospital) or referral department (if inside the hospital)] are presented in Tables S8,S9.
Adverse events/adverse reactions
This study is categorized as a retrospective observational study in which no individual case safety reporting applies. Based on the aggregated analysis of the data, no safety signals were reported nor identified for caplacizumab throughout the study.
Discussion
Although some publications include large numbers of TTP patients (25,26), only a few studies have evaluated the local disease burden, laboratory parameters, and treatment paradigm of iTTP in patients within the Arabian Gulf region (27,28). The primary objective of our study was to explore and identify the nature of iTTP and to assess the local burden (clinical and HRU) of the disease, as well as the management paradigm in the Greater Gulf region. The mean number of days observed to achieve platelet normalization (i.e., treatment response) was numerically lower for patients on caplacizumab compared with those who did not receive caplacizumab. The treatment response to caplacizumab therapy observed in our analysis is similar to the results reported in previous studies (29,30).
Before the advent of TPE, TTP was often fatal, with a mortality rate exceeding 90% (31). With a response rate of ~80% and a 10-year survival rate of >90%, TPE has revolutionized the therapeutic outcomes of TTP, demonstrating that an early and prompt diagnosis can improve the prognosis of TTP (31,32). Currently, TPE with or without corticosteroids is the frontline treatment option of iTTP and should be started promptly once the disorder is clinically suspected. However, TPE therapy does not significantly affect the associated neurological complications (33). While a regimen of TPE, corticosteroids, and rituximab was the initial treatment of choice in the majority of iTTP patients in the current study, other adjunctive therapies, such as monoclonal antibodies and new targeted therapies, were also used to enhance treatment response, achieve clinical remission, and prevent/delay disease relapse. After receiving TPE, a significant rise in platelet count was reported, indicating that treatment was effective. Clinical remission was achieved in almost 90% of the patients who received the TPE + corticosteroids + rituximab regimen, and this percentage reached 100% in patients who received this regimen plus caplacizumab. Our study’s caplacizumab treatment response rate is consistent with previous studies’ data (21,27). During our 6-month data collection period, the overall death rate among our patients was 10% (6 patients), and two deaths were unrelated to iTTP disease. Although caplacizumab was used in only 7 patients (11.7%), it was associated with 100% survival.
During the study period, troponin was the first marker to return to normal values (3.1 days), followed by creatinine (10.1 days) and LDH (24.0 days). LDH levels are known to fall concurrently with a reduction in hemolytic rate, making this clinical characteristic a useful indicator of the therapeutic response (34). This reported LDH level improvement, alongside the observed increase in platelet count, indicates resolving microangiopathy (30). Almost all patients in this study were hospitalized, and 60% of them needed ICU admission. TPE was available and provided to all patients, with a mean (SD) of 11.4 (9.9) sessions provided per patient. This reflects the availability of the initial treatment regimens and required healthcare resources in the Arabian Gulf region countries. Also, most ICU-admitted patients were new cases (97.2%), which may be attributed to such factors as diagnostic delay, inappropriate treatment, associated comorbidities, or unavailable advanced therapeutic options.
TTP develops due to ADAMTS13 (a vWF metalloprotease) suppression or deficiency, which slows down the breakdown of VWF multimers and leads to an increased number of ultra-large VWF multimers. In response, platelet adhesion and aggregation are activated, and small blood vessels will begin to fill with microthrombi (5). To confirm iTTP diagnosis, most physicians use ADAMTS13 activity level testing and anti-ADAMTS13 antibody testing, reflecting the critical data provided by ADAMTS13 for the diagnosis and differential diagnosis of iTTP. ADAMTS13 activity levels were measured in over 50% of patients, and 46.7% were assessed for ADAMTS13 inhibitory antibodies. Therefore, the clinical definition of a confirmed diagnosis of iTTP was the most reliable definition for most of our included patients.
More than half of ADAMTS13 tests (58.8%) were performed in laboratories outside the country. For these tests, the waiting time to receive the test result was 4 days more than that reported by local laboratories. Besides this diagnostic delay, such issues also add to the financial burden on patients and the local healthcare system because of the high number of staff involved, transportation logistics, sample preservation methods, and costs of the foreign laboratories. More than two-thirds of iTTP cases included in our analysis were referred from other hospitals due to unconfirmed diagnoses requiring specific assessment, unavailability of treatment resources, or even unavailability of required diagnostic tests. This high ratio of iTTP patient referrals has its merits and demerits; a patient’s referral indicates good use of resources, and that the patient is getting appropriate and required care within a timely manner (35). However, it also indicates that many hospitals in the healthcare system of the included countries need to be equipped with new diagnostic tools (such as ADAMTS13 enzyme testing) to provide a better healthcare service and lessen the diagnostic delay associated with iTTP. Additionally, it indicates a strong need for better resource allocation and increased healthcare expenditure on iTTP in the Arabian Gulf countries.
In Arabian Gulf countries, patient care for iTTP is influenced by several key factors that significantly shape the healthcare experience for affected individuals. Firstly, the healthcare system’s nature, encompassing both private and governmental hospitals, plays a crucial role. The insurance system further complicates matters, as Arabian Gulf countries have diverse insurance arrangements, from private health insurance to national healthcare coverage. The type of insurance a patient holds can profoundly affect their treatment access and financial coverage. Additionally, the cost of essential diagnostic tests like the ADAMTS13 activity test is a significant concern. Without comprehensive insurance coverage, patients may bear a substantial portion of these expenses, posing financial challenges for those dealing with iTTP. This impacts test affordability and can delay diagnosis and treatment, potentially leading to poorer outcomes. Therefore, understanding who covers the test costs—whether insurers, government programs, or patients themselves—is vital in comprehending the patient care landscape in Arabian Gulf countries.
Study limitations
The retrospective study design is an appropriate and scientifically robust method of collecting data that currently exists in patients’ medical records, but it has some limitations. Misclassification/ascertainment bias is one of the main limitations of a retrospective chart review, as retrospective studies depend on data entered into a clinical database and not collected for research. Investigators have limited control over the nature and quality of the obtained variables and cannot always ensure that the data of interest has been accurately and reliably recorded.
Selection bias is another restriction. In the present study, all available data were extracted, so the bias of excluding patients with more severe disease was avoided. Also, including multiple sites from different countries in the study ensured a geographically well-dispersed sample of sites, increasing the likelihood that the received data is representative. One notable limitation of this study pertains to the variability observed in PLASMIC and French TMA scores among the included patients. These scoring systems, while valuable tools for assisting in the diagnosis of iTTP, introduce a level of subjectivity into the process. The potential for different clinicians to interpret and assign scores differently may lead to misclassification, especially in cases with lower scores. Consequently, there is a risk of underestimating iTTP mortality rates and potentially skewing our understanding of the time required for patients to respond to treatment. This underscores the importance of recognizing the limitations associated with relying solely on scoring systems and highlights the need for ongoing efforts to standardize diagnostic criteria in iTTP, combining clinical judgment with scoring systems for more accurate patient classification and robust study conclusions.
Conclusions
TPE, corticosteroids, and rituximab were the most frequent therapies used in the management of iTTP in the Arabian Gulf region; however, other adjunctive therapies were used to enhance patients’ response, achieve clinical remission, and prevent/delay disease relapse. Despite not being used frequently in our study, caplacizumab was associated with a shorter time to platelet normalization. Most iTTP patients needed hospitalization and even ICU admission during their disease journey, indicating the huge burden of this disease on patients and local healthcare systems in the region. While ADAMTS13 enzyme-level testing alone is sufficient for the diagnosis of iTTP, the ADAMTS13 level and ADAMTS13 inhibitor profile should be considered after assessing the clinical features in order to increase the certainty of the diagnosis. All these real-world data will help to implement a better iTTP management plan and improve healthcare resource allocation in the Arabian Gulf and Middle East regions.
Acknowledgments
All authors are thankful to Dr. Ahmed Salah Hussein (Senior Medical Writer at RAY-CRO, Cairo, Egypt) and Dr. Mahmoud A. Ebada (Medical Writing Reviewing Lead at RAY-CRO, Cairo, Egypt) for their work in the medical writing of this manuscript and other related clinical documents. Also, the authors would like to extend their gratitude to Dr. Omnia Mokbel (Medical Information Manager at RAY-CRO, Cairo, Egypt), Dr. Omar M. Hussein (Medical Affairs Director at RAY-CRO, Cairo, Egypt) and Dr. Reham El Garhy (Vice President and General Manager at RAY-CRO, Cairo, Egypt) for their support and guidance. Editorial support (English language editing) was provided by Hanna Mourad-Agha, PhD, CMPP (Fishawack Communications Ltd., part of Avalere Health, USA) and was funded by Sanofi.
Funding: This work was sponsored by Sanofi KSA, which did not intervene in the design, implementation, data collection, writing, interpretation, preparation, and drafting of this document for publication. Sanofi’s team of experts has reviewed the final version of the manuscript. In addition, Sanofi funded the medical writing assistance of the clinical documents provided by RAY-CRO.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://aob.amegroups.com/article/view/10.21037/aob-23-29/rc
Data Sharing Statement: Available at https://aob.amegroups.com/article/view/10.21037/aob-23-29/dss
Peer Review File: Available at https://aob.amegroups.com/article/view/10.21037/aob-23-29/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-23-29/coif). A.M., M.R. and Z.C. are employees of Sanofi Genzyme, and may hold shares or stock options in the company. M.N. was an employee of Sanofi at the time the manuscript was in preparation. All authors report that this work was sponsored by Sanofi KSA, which did not intervene in the design, implementation, data collection, writing, interpretation, preparation, and drafting of this document for publication. Sanofi’s team of experts has reviewed the final version of the manuscript. In addition, Sanofi funded the medical writing assistance of the clinical documents provided by RAY-CRO. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the institutional review board of participated countries. In Saudi Arabia, IRB approvals were obtained from Jeddah Health Affairs under the IRB KACST, KSA (No. H-02-J-002, on 12/04/2021) and from King Saud University under the IRB KACST (Registration No. H-01-R-002, on 23/08/2021). In Oman, IRB approval was obtained from the Ministry of Health (MOH/CSR/21/24592, on 04/08/2021). In UAE, approval was obtained from Tawam Hospital (DOH/CVDC/2021/654 SKMC, on 20/05/2021). In Qatar, this study was approved by the National Centre for Cancer Care and Research (Protocol No. MRC-02-21-725). In Kuwait, the study was approved by the Ministry of Health (No.: 1709 / 2020). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sukumar S, Lämmle B, Cataland SR. Thrombotic Thrombocytopenic Purpura: Pathophysiology, Diagnosis, and Management. J Clin Med 2021;10:536. [Crossref] [PubMed]
- Moake JL. Thrombotic microangiopathies. N Engl J Med 2002;347:589-600. [Crossref] [PubMed]
- Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood 2017;129:2836-46. [Crossref] [PubMed]
- Furlan M, Robles R, Galbusera M, et al. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med 1998;339:1578-84. [Crossref] [PubMed]
- Tsai HM. Pathophysiology of thrombotic thrombocytopenic purpura. Int J Hematol 2010;91:1-19. [Crossref] [PubMed]
- Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med 1998;339:1585-94. [Crossref] [PubMed]
- Ezra Y, Rose M, Eldor A. Therapy and prevention of thrombotic thrombocytopenic purpura during pregnancy: a clinical study of 16 pregnancies. Am J Hematol 1996;51:1-6. [Crossref] [PubMed]
- Onundarson PT, Rowe JM, Heal JM, et al. Response to plasma exchange and splenectomy in thrombotic thrombocytopenic purpura. A 10-year experience at a single institution. Arch Intern Med 1992;152:791-6. [Crossref] [PubMed]
- Sayani FA, Abrams CS. How I treat refractory thrombotic thrombocytopenic purpura. Blood 2015;125:3860-7. [Crossref] [PubMed]
- Willis MS, Bandarenko N. Relapse of thrombotic thrombocytopenic purpura: is it a continuum of disease? Semin Thromb Hemost 2005;31:700-8. [Crossref] [PubMed]
- Scully M, Yarranton H, Liesner R, et al. Regional UK TTP registry: correlation with laboratory ADAMTS 13 analysis and clinical features. Br J Haematol 2008;142:819-26. [Crossref] [PubMed]
- Mariotte E, Azoulay E, Galicier L, et al. Epidemiology and pathophysiology of adulthood-onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): a cross-sectional analysis of the French national registry for thrombotic microangiopathy. Lancet Haematol 2016;3:e237-45. [Crossref] [PubMed]
- Miesbach W, Menne J, Bommer M, et al. Incidence of acquired thrombotic thrombocytopenic purpura in Germany: a hospital level study. Orphanet J Rare Dis 2019;14:260. [Crossref] [PubMed]
- Martino S, Jamme M, Deligny C, et al. Thrombotic Thrombocytopenic Purpura in Black People: Impact of Ethnicity on Survival and Genetic Risk Factors. PLoS One 2016;11:e0156679. [Crossref] [PubMed]
- Reese JA, Muthurajah DS, Kremer Hovinga JA, et al. Children and adults with thrombotic thrombocytopenic purpura associated with severe, acquired Adamts13 deficiency: comparison of incidence, demographic and clinical features. Pediatr Blood Cancer 2013;60:1676-82. [Crossref] [PubMed]
- Lee W, Perimbeti S, Vazquez Martinez M, et al. Higher Incidence of TTP in African Americans and Females: An Analysis of Demographics, Cost and Length of Stay in Teaching and Nonteaching Hospitals for Thrombotic Thrombocytopenic Purpura Between 1999 and 2013. Blood 2016;128:4735. [Crossref]
- Zheng XL, Vesely SK, Cataland SR, et al. ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura. J Thromb Haemost 2020;18:2486-95. [Crossref] [PubMed]
- Kempton CL. Thrombotic thrombocytopenic purpura. In: Hillyer CD, Shaz BH, Zimring JC, et al. editors. Transfusion Medicine and Hemostasis: Clinical and Laboratory Aspects. Academic Press; 2009:509-13.
- Plautz WE, Raval JS, Dyer MR, et al. ADAMTS13: origins, applications, and prospects. Transfusion 2018;58:2453-62. [Crossref] [PubMed]
- Chang JC. TTP-like syndrome: novel concept and molecular pathogenesis of endotheliopathy-associated vascular microthrombotic disease. Thromb J 2018;16:20. [Crossref] [PubMed]
- Peyvandi F, Callewaert F. Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura. N Engl J Med 2016;374:2497-8. [Crossref] [PubMed]
- Elston DM. The Hawthorne effect. J Am Acad Dermatol 2021;S0190-9622(21)00245-0.
- Rabea M, Nagib M, Chouikrat Z. Acquired thrombotic thrombocytopenic purpura landscaping in Gulf countries: a study protocol. IJMDC 2022;6:900-8. [Crossref]
- National Organization for Rare Disorders. Thrombotic Thrombocytopenic Purpura. Available online: https://rarediseases.org/rare-diseases/thrombotic-thrombocytopenic-purpura/
- George JN, Vesely SK, Terrell DR. The Oklahoma Thrombotic Thrombocytopenic Purpura-Hemolytic Uremic Syndrome (TTP-HUS) Registry: a community perspective of patients with clinically diagnosed TTP-HUS. Semin Hematol 2004;41:60-7. [Crossref] [PubMed]
- Deng MY, Zhang GS, Zhang Y, et al. Analysis of clinical and laboratory characteristics in 42 patients with thrombotic thrombocytopenic purpura from a single center in China. Transfus Apher Sci 2013;49:447-52. [Crossref] [PubMed]
- Iqbal S, Zaidi SZ, Motabi IH, et al. Thrombotic thrombocytopenic purpura - analysis of clinical features, laboratory characteristics and therapeutic outcome of 24 patients treated at a Tertiary Care Center in Saudi Arabia. Pak J Med Sci 2016;32:1494-9. [Crossref] [PubMed]
- Alqaraawi A, Owaidah T, Alenzai A, et al. Acquired Deficiency of Von Willebrand Factor- Cleaving Protease in an HIV-Infected Patient with Relapsing Thrombotic Thrombocytopenic Purpura. Journal of Applied Hematology 2012;3:86-8.
- Scully M, Cataland SR, Peyvandi F, et al. Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura. N Engl J Med 2019;380:335-46. [Crossref] [PubMed]
- Völker LA, Brinkkoetter PT, Knöbl PN, et al. Treatment of acquired thrombotic thrombocytopenic purpura without plasma exchange in selected patients under caplacizumab. J Thromb Haemost 2020;18:3061-6. [Crossref] [PubMed]
- Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood 2008;112:11-8. [Crossref] [PubMed]
- Bell WR, Braine HG, Ness PM, et al. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med 1991;325:398-403. [Crossref] [PubMed]
- Scully M, Hunt BJ, Benjamin S, et al. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol 2012;158:323-35. [Crossref] [PubMed]
- Kaiser C, Gembruch U, Janzen V, et al. Thrombotic thrombocytopenic purpura. J Matern Fetal Neonatal Med 2012;25:2138-40. [Crossref] [PubMed]
- Hartveit M, Vanhaecht K, Thorsen O, et al. Quality indicators for the referral process from primary to specialised mental health care: an explorative study in accordance with the RAND appropriateness method. BMC Health Serv Res 2017;17:4. [Crossref] [PubMed]
Cite this article as: Al Rasheed M, Alsayegh F, Al Mohareb F, Aljatham AA, Alqahtani FH, Malhan H, Osman HY, Aal-Yaseen H, Salama H, Al Saeed HH, Al-Tourah L, Sallam M, Marashi M, Qari M, Hosseini MO, Al-Khabori M, Shalaby N, Kashari OF, Taha RY, Alhashami S, Mekky A, Rabea M, Naguib M, Chouikrat Z. Immune-mediated thrombotic thrombocytopenic purpura landscaping in Gulf countries: a real-world evidence study (ATHENA Study). Ann Blood 2024;9:3.