
Sticky platelet syndrome—diagnostic issues and controversies
Background
Sticky platelet syndrome (SPS) was first described in 1983 by Holiday et al. (1) in a group of young people suffering from ischemic stroke; some of them also had a history of migraine. History of early myocardial infarction, migraine, or ischemic stroke in their relatives was often present and enhanced aggregation of platelets was described in these patients. In 1984, Mammen et al. treated a young pregnant woman suffering from myocardial infarction with no atherosclerotic changes of her coronary arteries and increased platelet aggregation. In 1988, Mammen et al. described patients and families with thrombosis (predominantly arterial) and platelet hyperaggregability (2). The concentrations of adenosine diphosphate (ADP) at 2.34, 1.17, and 0.58 µM and concentrations of epinephrine at 11, 1.1, and 0.55 µM have been used for aggregation induction using light transmission aggregometry (LTA). A relationship between the concentration of inductor and maximum amplitude of the aggregation curve was observed in healthy controls, whereas no significant decline of maximal aggregation was observed in patients with SPS, when decreasing concentration of inductors were used (3). Two forms of SPS were defined: type I characterized by hyperaggregability induced by ADP and epinephrine, and type II characterized by hyperaggregability induced by epinephrine only. Type III characterized by hyperaggregability induced by ADP only was described by Bick (4) and the diagnostic criteria have been established. Hyperaggregability was defined as aggregation amplitude above the upper range of normal values, which were calculated from a group of healthy volunteers. These diagnostic criteria have been used in most publications up to the present time (5-9).
Numerous case reports and cohort studies were published suggesting the relationship of platelet hyperaggregability with arterial and venous thromboembolism, retinal vessel thrombotic occlusion, thrombotic posttransplant complications, and recurrent miscarriage (4-9). However, conflicting results of a large prospective study were published as well. In Framingham study, the platelet aggregation at ADP concentration of 1.0 µM was significantly associated with incident myocardial infarction/stroke in a multivariable model during follow-up (median 20.4 years), but no association of platelet hyperreactivity to low concentrations of epinephrine with incidence of myocardial infarction/stroke was found (10). These results are not in contradiction with the concept of SPS, because the average age of participants at the beginning of the study was 54.3 years and the significance of other factors contributing to the atherothrombosis increases with increasing age.
The research has been focused on pathophysiological aspects of SPS in recent decade, resulting in some new insights. The question of spontaneous activation of platelets in patients with SPS was answered, when higher expression of P-selectin, CD63, and CD51 on platelet surface in patients with SPS was described (11). The question of type of inheritance has also been repeatedly discussed. SPS is traditionally considered as an autosomal dominant hereditary disorder. However, despite huge efforts the studies failed to find unique specific gene responsible for the hyperaggregability of platelets; the association of various polymorphisms with enhanced platelet aggregability was reported, and possible polygenic type of SPS heredity has been suggested (12,13).
The rationale of the article is to summarize the diagnostic controversies and the objective is to suggest the solution of them.
Despite increasing publication rate, many experts in hemostasis have doubts about SPS existence. They often argue that many healthy persons had “sticky platelets” when they were investigated in their lab. The experts, who believe in existence of SPS argue that it is a problem of inappropriate handling of the sample. In fact, the investigation of platelets aggregation requires fresh material and highly meticulous sample collection and handling (14). The standardization of the preanalytical and analytical phase seems thus to be the cornerstone of further advance in this debate. The feasibility of universal standardization of the preanalytical phase is nevertheless limited because the organization of sample collection and transport is different in particular hospitals and clinics. The effort should be thus focused on maximal standardization of the process in every unique hospital or clinic, and the statistical tools will be used for further analyses.
Moreover, many other questions concerning the diagnostic criteria are to be answered; some of these questions are discussed in the following text.
Is one result sufficient?
The knowledge of repeatability and reproducibility is crucial for the answer to this question.
According to the original diagnostic criteria, repeated testing is required only in the case when hyperaggregability to one concentration of one reagent is measured and repeated testing is not required when hyperaggregability to two concentrations of one reagent or to one concentration of both reagents is detected. There are, however, strong arguments against the validity of unrepeated testing. The reports on repeatability and reproducibility studies are sparse.
Repeatability of aggregation maximal amplitude using ADP 5 µM [coefficient of variation (CV) 4.15%] and epinephrine 5.4 µM (CV 6.42%) was reported as excellent when measured using the coagulation analyzer Sysmex CS-2100i (15). Our results are less encouraging. We measured the samples of eight healthy blood donors; each sample was measured simultaneously in four aggregation channels of the coagulation analyzer Sysmex CS-2500 with the same concentration of the inductor. The concentrations of epinephrine at 10, 1, and 0.5 µM and the concentrations of ADP at 2.5, 1, and 0.5 µM were used. The CV were calculated from the results of these four simultaneous measurements; they are summarized in Table 1.
Table 1
| No. | ADP | Epinephrine | |||||
|---|---|---|---|---|---|---|---|
| 2.5 µM | 1.0 µM | 0.5 µM | 10.0 µM | 1.0 µM | 0.5 µM | ||
| 1 | 0.034 | 0.255 | 0.060 | 0.027 | 0.038 | 0.379 | |
| 2 | 0.030 | 0.070 | 0.158 | 0.023 | 0.029 | 0.025 | |
| 3 | 0.065 | 0.151 | 0.132 | 0.044 | 0.031 | 0.039 | |
| 4 | 0.190 | 0.132 | 0.179 | 0.066 | 0.055 | 0.028 | |
| 5 | 0.060 | 0.474 | 0.070 | 0.029 | 0.128 | 0.175 | |
| 6 | 0.073 | 0.586 | 0.106 | 0.050 | 0.044 | 0.308 | |
| 7 | 0.064 | 0.184 | NA | 0.428 | 0.643 | 0.263 | |
| 8 | 0.100 | 0.119 | 0.335 | 0.038 | 0.104 | 0.248 | |
The CV is higher than 20% in 21% of simultaneous measurements. Repeated testing is thus required. CV, coefficient of variation; ADP, adenosine diphosphate.
The CV exceeded 20% in 10/47 (21%) of results. Despite the weakness of this study (only four simultaneous measurements used for CV determination), our results suggest the necessity of repeated testing.
Reproducibility study of aggregation with epinephrine 0.4 µM has been published as a part of a large article in Blood (16). Twenty-seven healthy volunteers have been tested repeatedly over four consecutive weeks. In 23 of 27 (85%) subjects, the results were extremely consistent, especially in those who exhibited low aggregability. Nine (9/27, 33%) persons demonstrated hyperaggregation (maximum amplitude greater than 60%) on at least one occasion, and most of them (seven subjects) demonstrated hyperaggregability on at least two occasions and in five of them the hyperaggregability was detected on all four occasions. Based on this study, the low aggregability detected seems to be sufficient for exclusion of the hyperaggregability, while when hyperaggregability or borderline aggregability is detected, repeated examination is required. Repeated testing is recommended also for diagnosis of functional defects of platelets (17).
Is pre-analytical phase important?
As in any other laboratory test, the pre-analytical phase is crucial here to obtain relevant results. In this case is very important the education of investigated persons regarding factors, which may influence the platelet function and aggregometry results, such as antiplatelet drugs, nonsteroidal anti-inflammatory drugs, fish oil, and herbal products. They should be encouraged to stop smoking in the evening before blood collection. The effect of pregnancy, recent thromboembolism, recent trauma, infection and any recent stressful event must be avoided at the time of blood sampling.
Are the concentrations of inductors appropriate?
The concentrations of ADP at 2.34, 1.17, and 0.58 µM and concentrations of epinephrine at 11, 1.1, and 0.55 µM have been traditionally used for SPS diagnosis, and similar concentrations (ADP concentrations at 2.5, 1, and 0.5 µM and epinephrine concentrations at 10, 1, and 0.5 µM) are also used in some labs. The reasons for using these concentrations are probably based on the tradition rather than on scientific principles. The knowledge of distribution of values in healthy population is essential to solve the problem of appropriateness currently used concentrations of inductors. The distribution of aggregation response to epinephrine concentrations at 0.4, 1.5, and 10 µM among a large group of 359 healthy volunteers was investigated (16). At the epinephrine concentration of 0.4 µM, the majority of subjects exhibited low platelet aggregation (<40%) while in 14% of persons the aggregation was greater than 60%. This epinephrine concentration could be thus considered as suitable for distinction between the minority subjects with clearly hyperreactive phenotype and majority of subjects with “usual” platelet aggregability. With increasing epinephrine concentration, the proportion of subjects with aggregation >60% increased and the concentrations of epinephrine 1.5, and 10 µM are thus appropriate for division of the population into low responders and high responders rather than for detection of hyperaggregability in terms of unusually enhanced aggregation. The distribution of values was not normal (Gaussian) when any epinephrine concentration was used (16). Our results were similar. We investigated 100 healthy volunteers using ADP concentrations of 2.5, 1, and 0.5 µM, and epinephrine concentrations of 10, 2.5, 1, and 0.5 µM. The distribution of values was also not normal (Gaussian) when any concentration of epinephrine and ADP was used. The concentrations of epinephrine at 1 and 0.5 µM, and concentrations of ADP at 1 and 0.5 µM were recognized as suitable for diagnosis of “hyperreactive phenotype” (Figure 1). However, concentrations of epinephrine at 10 and 2.5 µM and concentration of ADP at 2.5 µM were found as inappropriate (Figure 2).
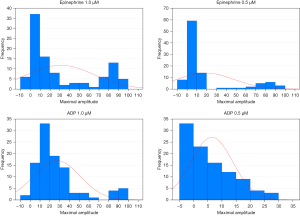
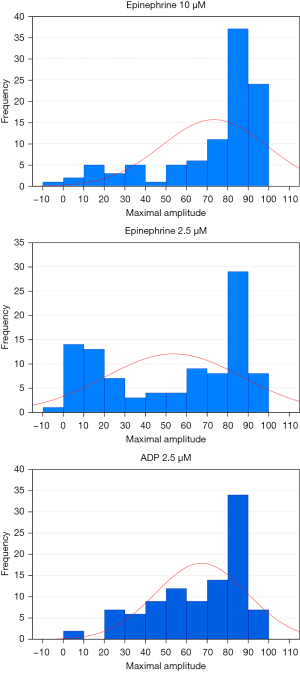
Is the platelet aggregability age dependent?
No relation between age and aggregation induced by ADP, collagen and epinephrine was observed in Japanese population (18), while the platelet aggregatory response to ADP and collagen markedly increased with age in healthy people in Germany (19). In elderly healthy people (≥70 years) the aggregation response to ADP was enhanced comparing with the middle-aged (40 to 55 years) and with young (20 to 30 years) people. The elderly also demonstrated higher P2Y12 receptor density, and expression of P-selectin (20). Because the investigation of thrombophilia is considered to be useful for subjects younger than 45 years, the question of age-dependency of hyperaggregability is relevant to the young and middle-aged people. The results of a group of 359 healthy volunteers (age 34.2±10.4 years) revealed no age-dependency of aggregation induced by epinephrine concentration 0.4 µM (16). No correlation of aggregation response to ADP concentrations at 2.5, 1, and 0.5 µM, and epinephrine concentrations at 10, 2.5, 1, and 0.5 µM with age in a group of 100 healthy volunteers 20 to 50 years old was observed in our lab (Table 2).
Table 2
| Inductor | Spearman coefficient R | P |
|---|---|---|
| Epinephrine | ||
| 10.0 µM | 0.005 | 0.95 |
| 2.5 µM | −0.002 | 0.98 |
| 1.0 µM | 0.022 | 0.82 |
| 0.5 µM | −0.012 | 0.90 |
| ADP | ||
| 2.5 µM | −0.070 | 0.48 |
| 1.0 µM | −0.094 | 0.34 |
| 0.1 µM | −0.016 | 0.87 |
No correlations of aggregation amplitude with age were found. ADP, adenosine diphosphate.
Is the platelet aggregability dependent on gender?
In a small study, female platelets had increased aggregation with ADP stimulation, as compared to male platelets (21). Negative correlations of testosterone level and platelet aggregation induced by ADP 10 µM, collagen 1 µg/mL, and arachidonic acid 0.5 mM were described (22). The aggregation >60% to epinephrine concentration 0.4 µM was found to be more frequent in women than in men (16). We found the hyperaggregability >80% induced by epinephrine 0.5 µM, and by ADP 2.5 and 1 µM more frequent in women than in men. In summary, women are more prone to platelet hyperaggregation when low concentrations of inductors are used. If the reference ranges were important for the diagnostic criteria, they should be calculated separately for men and for women. Fortunately, the cut-offs are probably not identical with the upper limits of reference ranges, as described below.
Is the upper limit of reference range an appropriate cut-off?
The cut-offs, used for establishment of traditional SPS diagnostic criteria, were determined as the upper limits of reference ranges for particular concentrations of inductors. Nevertheless, there exist at least two controversial issues concerning the appropriateness of this approach. First, the distribution of values is not Gaussian and calculation of reference ranges requires more measurements than in case of Gaussian distribution. The number of healthy controls published by Mammen was too low for calculation of reference ranges in case of non-Gaussian distribution of values (3).
Second, the reference ranges are defined as the interval between which 95% of values of a reference population fall into, while cut-off value is generally used for confirmation or exclusion of the diagnosis with prespecified probability. It is dependent on the prevalence of the disorder, on the distributions of values in the healthy population and in the population of patients, and on the uncertainty of measurement. The cut-off value could be identical with the upper reference range limit only in the case when the prevalence of the disease would be 2.5%. Based on the fact, that only a minority of subjects demonstrated aggregation within range 40% to 60%, some authors suggested that less than 40% and more than 60% represent appropriate criteria for distinguishing between little or absent aggregation and full or complete aggregation (16). In fact, because of unknown SPS prevalence in the population, the doubts about appropriateness of historically established and traditionally used cut-off values are plausible.
Is the LTA an optimal method for platelet hyperaggregability evaluation?
There are many pitfalls associated with the LTA, and alternative approaches to the evaluation of platelet hyperaggregability should be discussed. Some methods such as PFA-100/200, VerifyNow, and Multiplate, are very user-friendly, however these analyzers are constructed for specific purposes and we have very sparse, if any, data about their use for detection of hyperaggregability related to the risk of thrombosis. More sophisticated and multifunctional methods were sometimes used for detection of platelet hyperaggregability. The flow cytometric evaluation of the activation related antigens expression on unstimulated platelets seems to be a promising method, but the flow cytometry is even less available, than LTA. The results of impedance aggregometry and LTA were compared in our lab two decades ago, and the results of LTA were found to be more robust than the results of impedance aggregometry.
Conclusions
The SPS was described 40 years ago. Despite accruing publication rate, some doubts persist and new insights into this topic are emerging. A reappraisal of traditional diagnostic criteria seems to be required. Because of a suboptimal repeatability and reproducibility in some cases, the investigation should be repeated, at least in the case of borderline results. The traditionally used concentrations of epinephrine 10 µM, and concentration of ADP 2.5 µM seem to be not “low enough” for detection of hyperaggregability with low concentrations of these inductors, because the aggregation amplitude above 80% is observed in samples obtained from a significant proportion of healthy volunteers. The concentrations of epinephrine and ADP at 1.0 and 0.5 µM are probably more suitable. The upper limits based on the investigation of healthy population, are probably not identical with the cut-offs used as SPS diagnostic criteria, and the appropriate cut-offs will be established based on further clinical studies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Peter Kubisz and Pavol Holly) for the series “Sticky Platelet Syndrome” published in Annals of Blood. The article has undergone external peer review.
Peer Review File: Available at https://aob.amegroups.com/article/view/10.21037/aob-22-49/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-22-49/coif). The series “Sticky Platelet Syndrome” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Holiday PL, Mammen E, Gilroy J. Sticky platelet syndrome and cerebral infarction in young adults. Proceedings of the Ninth International Joint Conference on Stroke and Cerebral Circulation; Phoenix, Arizona; 1983.
- Mammen EF, Barnhart MI, Selik NR, et al. "Sticky platelet syndrome": a congenital platelet abnormality predisposing to thrombosis? Folia Haematol Int Mag Klin Morphol Blutforsch 1988;115:361-5. [PubMed]
- Mammen E. Ten Years’ Experience with the “Sticky Platelet Syndrome”. Clin Appl Thromb Hemost 1995;1:66-72. [Crossref]
- Bick RL. Sticky Platelet Syndrome: a Common Cause of Unexplained Arterial and Venous Thrombosis. Clin Appl Thromb Hemost 1998;4:77-81. [Crossref]
- Šimonová R, Bartosová L, Chudy P, et al. Nine kindreds of familial sticky platelet syndrome phenotype. Clin Appl Thromb Hemost 2013;19:395-401. [Crossref] [PubMed]
- Tekgündüz E, Demir M, Akyol Erikçi A, et al. Sticky platelet syndrome in patients with uninduced venous thrombosis. Turk J Haematol 2013;30:48-52. [PubMed]
- El-Amm JM, Andersen J, Gruber SA. Sticky platelet syndrome: a manageable risk factor for posttransplant thromboembolic events. Am J Transplant 2008;8:465. [Crossref] [PubMed]
- Azamar-Solis B, Cantero-Fortiz Y, Olivares-Gazca JC, et al. Primary Thrombophilia in Mexico XIII: Localization of the Thrombotic Events in Mexican Mestizos With the Sticky Platelet Syndrome. Clin Appl Thromb Hemost 2019;25:1076029619841700. [Crossref] [PubMed]
- Yagmur E, Bast E, Mühlfeld AS, et al. High Prevalence of Sticky Platelet Syndrome in Patients with Infertility and Pregnancy Loss. J Clin Med 2019;8:1328. [Crossref] [PubMed]
- Puurunen MK, Hwang SJ, Larson MG, et al. ADP Platelet Hyperreactivity Predicts Cardiovascular Disease in the FHS (Framingham Heart Study). J Am Heart Assoc 2018;7:e008522. [Crossref] [PubMed]
- Staško J, Bartošová L, Mýtnik M, et al. Are the platelets activated in sticky platelet syndrome? Thromb Res 2011;128:96-7. [Crossref] [PubMed]
- Sokol J, Skerenova M, Ivankova J, et al. Association of Genetic Variability in Selected Genes in Patients With Deep Vein Thrombosis and Platelet Hyperaggregability. Clin Appl Thromb Hemost 2018;24:1027-32. [Crossref] [PubMed]
- Sokol J, Skerenova M, Biringer K, et al. Genetic variations of the GP6 regulatory region in patients with sticky platelet syndrome and miscarriage. Expert Rev Hematol 2015;8:863-8. [Crossref] [PubMed]
- Favaloro EJ, Lippi G. Commentary: Controversies in Thrombosis and Hemostasis Part 2-Does Sticky Platelet Syndrome Exist? Semin Thromb Hemost 2019;45:69-72. [Crossref] [PubMed]
- Ling LQ, Liao J, Niu Q, et al. Evaluation of an automated light transmission aggregometry. Platelets 2017;28:712-9. [Crossref] [PubMed]
- Yee DL, Sun CW, Bergeron AL, et al. Aggregometry detects platelet hyperreactivity in healthy individuals. Blood 2005;106:2723-9. [Crossref] [PubMed]
- Ghosh K, Nair S, Kulkarni B, et al. Platelet function tests using platelet aggregometry: need for repetition of the test for diagnosis of defective platelet function. Platelets 2003;14:351-4. [Crossref] [PubMed]
- Maruyama Y. Age differences in platelet aggregation, plasma levels of prostanoid, cyclic nucleotide and lipids in Japanese. Nihon Ronen Igakkai Zasshi 1989;26:165-73. [Crossref] [PubMed]
- Terres W, Weber K, Kupper W, et al. Age, cardiovascular risk factors and coronary heart disease as determinants of platelet function in men. A multivariate approach. Thromb Res 1991;62:649-61. [Crossref] [PubMed]
- Gnanenthiran SR, Pennings GJ, Reddel CJ, et al. Identification of a Distinct Platelet Phenotype in the Elderly: ADP Hypersensitivity Coexists With Platelet PAR (Protease-Activated Receptor)-1 and PAR-4-Mediated Thrombin Resistance. Arterioscler Thromb Vasc Biol 2022;42:960-72. [Crossref] [PubMed]
- Coleman JR, Moore EE, Kelher MR, et al. Female platelets have distinct functional activity compared with male platelets: Implications in transfusion practice and treatment of trauma-induced coagulopathy. J Trauma Acute Care Surg 2019;87:1052-60. [Crossref] [PubMed]
- Karolczak K, Konieczna L, Kostka T, et al. Testosterone and dihydrotestosterone reduce platelet activation and reactivity in older men and women. Aging (Albany NY) 2018;10:902-29. [Crossref] [PubMed]
Cite this article as: Kessler P, Peliskova L, Prokopova J. Sticky platelet syndrome—diagnostic issues and controversies. Ann Blood 2024;9:10.


