Economics of patient blood management in the United States: a narrative review
Introduction
Patient blood management (PBM) is a multidisciplinary approach to optimize the use of blood and blood products in clinical practice. The economic impact of implementing a PBM program has gained attention in recent years, as healthcare costs continue to rise and there is growing awareness of the need to reduce unnecessary transfusions. This review aims to explore the economics of PBM programs by examining the cost-effectiveness of PBM strategies, as well as the potential cost savings associated with reducing blood product utilization.
To provide a thorough understanding of the background and context of PBM programs, it is important to discuss their origins and evolution. The need for better PBM was recognized as the average age of the donor population increased and blood shortages became more common. At the same time, bloodless surgery programs were initiated to better serve patients who refused blood transfusion; thus the ability to provide medical care with fewer transfusions was demonstrated. PBM has emerged as an evidence-based approach to transfusion management that encompasses a range of interventions, including preoperative optimization, intraoperative blood conservation techniques, and postoperative anemia management. Studies have shown that the implementation of PBM strategies can improve patient outcomes, reduce the need for transfusions, and lower healthcare costs.
Franchini et al. published a systematic review in 2019 aimed at evaluating the cost-effectiveness of PBM strategies (1). They included 13 studies that compared the cost associated and clinical outcomes of PBM strategies to standard care. It was found that PBM programs were generally cost-effective, with some interventions being more cost-effective than others. It was noted that there was a lack of high-quality studies and further research is needed. In addition, Bolcato et al. in 2020 discussed in a scoping review 60 studies that examined the benefit and costs of PBM interventions (2). They found that while there was a growing body of literature on the economics of PBM, there was a lack of standardized economic evaluation methods, making it difficult to compare studies and draw conclusions on beneficence.
Together, these two reviews highlight the need for further research on the economics of PBM programs. While there is some evidence to suggest that PBM interventions can be cost-effective, there is still a lack of high-quality studies and standardized methods for economic evaluation. We present this article in accordance with the Narrative Review reporting checklist (available at https://aob.amegroups.com/article/view/10.21037/aob-22-35/rc).
Methods
Search strategies
A literature search was performed to identify articles on the economics of PBM (Table 1). The search was conducted in April 2022 and updated in October 2023 to include any relevant articles published since the initial search. The following electronic databases were searched: PubMed, Embase, and Scopus. The search strategy included the following keywords: “patient blood management”, “blood transfusion”, “cost”, “economic analysis”, and “United States”. The search was limited to articles published in English language.
Table 1
| Items | Specification |
|---|---|
| Date of search | April 2022 and updated in October 2023, no end date specified in search. Studies and data were chosen by relevance and where applicable, most recent study was used |
| Databases and other sources searched | PubMed, Embase, and Scopus |
| Search terms used | “patient blood management”, “blood transfusion”, “cost”, “economic analysis”, and “United States” |
| Timeframe | Publications before October 2023 |
| Inclusion and exclusion criteria | Studies were included if they focused on the economics of patient blood management. Studies that focused on non-economic aspects of blood management were excluded. Additionally, studies that did not provide information on costs, or that were not published in English language, were excluded |
| Selection process | Selection was conducted independently by each author. Results were shared amongst the group |
Inclusion and exclusion criteria
Studies were included if they focused on the economics of PBM. Studies that focused on non-economic aspects of blood management were excluded. Additionally, studies that did not provide information on costs, or that were not published in English language, were excluded.
Data extraction
Data were extracted from each study on the following variables: study design, study population, interventions, outcomes, and costs.
Data analysis
Data were analyzed using a narrative synthesis approach. Results were summarized and presented in tabular and graphical format.
Blood component overview
Whole blood is collected from donors and processed through various means to create individual blood components for use, such as: red blood cells (RBCs), plasma, platelets, and cryoprecipitate (3). Separating whole blood into multiple components allows more than one patient to benefit from a single donated unit, makes it possible for patients to get only the component required for adequate treatment, and allows for compatible, but not type-specific, products to be given.
RBCs can be prepared by removing approximately 80% of plasma from a unit of whole blood or by collecting through an apheresis donation (3). They are indicated when increasing the blood’s oxygen carrying capacity is necessary to maintain normal tissue oxygenation (4). These include situations of uncompensated anemia such as acute blood loss because of trauma or surgery, and chronic anemia due to blood disorders or slow, prolonged bleeding. RBCs can be washed, leukoreduced, or irradiated depending on patient need.
Few studies have been done, either in the U.S. or elsewhere, that discuss the costs of blood transfusion. The first point of discussion needs to be what should be included when attempting to do such a cost accounting. In 2003, members of the Society for the Advancement of Blood Management convened a consensus conference where they discussed everything that goes into providing a blood transfusion (5). Their publication provides an excellent framework to begin that discussion.
Direct costs of blood components involve blood collection containers and labels, sample tubes used to collect donor blood for various tests, and the reagents and supplies needed to perform the testing. Indirect costs of collection include the costs to recruit donors (including providing incentives) and to provide an environment where the blood can be collected, including information systems, donor chairs, environmental controls, and materials such as stickers, printer paper, and refreshments for donors. There are costs incurred for transporting blood from where it is collected to where it is tested, processed and stored, and later, to the location where it will be transfused. Indirect costs of testing similarly include environmental controls and information system costs, but also costs for maintenance of testing equipment and storage devices. Other direct costs, depending on the type of component produced might include leukocyte reduction filters and pathogen-reduction technology (6). After the blood is tested, the units are labelled and stored, which again incurs direct costs (e.g., labels) and indirect costs (storage and information systems). Beyond these costs already listed, the most expensive aspect of the blood collection process is the cost of paying trained staff to do the front-line work and comply with all regulatory expectations. Examples of direct and indirect costs are provided in Table 2. To calculate the overall cost for a unit of blood, the direct costs are added together, and an amount is calculated for each indirect cost, which is then added to the direct costs. As an example, if a blood center spends $10,000/month on personnel wages and benefits plus $3,000/month on supplies (e.g., testing reagents and donor refreshments) plus $1,000/month on utilities and equipment maintenance plus an average of $1,000/month for depreciation of its assets, it would have $15,000/month in indirect costs. If it collects 100 apheresis packed red cell units in that month (and nothing else), the indirect cost for each unit would be $150. Direct costs for each unit might be $40 for the blood bag and $2 for the label. The blood center’s total cost for each unit would then be $192.
Table 2
| Location | Direct costs | Indirect costs |
|---|---|---|
| Blood center (donor-related) | Collection containers | Donor recruitment |
| Labels | Donor chairs | |
| Specimen tubes | Information systems | |
| Testing reagents | Refreshments | |
| Testing supplies | Testing instruments | |
| Blood center (component processing) | Labels | Storage device maintenance |
| Leukoreduction filters | Information systems | |
| Pathogen-reduction equipment | Environmental controls | |
| Transport boxes | Personnel | |
| Hospital (transfusion service) | Charge from blood supplier for each unit | Storage device maintenance |
| Testing reagents and supplies | Information systems | |
| Transfer bags | Environmental controls | |
| Aliquot syringes | Personnel | |
| Hospital (blood administration) | Intravenous catheters | IV pump and blood warmer maintenance |
| Infusion sets | Information systems | |
| Compatible intravenous fluids | Environmental controls | |
| Medications (if needed to avoid transfusion reactions) | Personnel |
IV, intravenous.
Blood collection centers calculate these costs and use them to determine the charge of each type of component they produce. When discussing reimbursement for these components, it is important to bear in mind that these prices reflect the direct and indirect costs of everything necessary to produce each component, not the cost of the component itself. Based on data published from the 2019 National Blood Collection and Utilization Survey (NBCUS), the median and mean costs of units purchased from blood suppliers are listed in Table 3 (7).
Table 3
| Component | Median price ($) | Mean price ($) | IQR ($) |
|---|---|---|---|
| Leukoreduced RBC | 208 | 215 | 198–215 |
| Leukoreduced apheresis platelets | 516 | 520 | 491–543 |
| Pathogen-reduced apheresis platelets | 617 | 610 | 570–659 |
| Fresh frozen plasma | 50 | 53 | 43–59 |
| Plasma frozen within 24 hours | 50 | 53 | 43–58 |
| Cryoprecipitated AHF | 51 | 55 | 42–62 |
NBCUS, National Blood Collection and Utilization Survey; IQR, interquartile range; RBC, red blood cell; AHF, antihemophilic factor.
The charge set by the blood center, in turn, is the starting direct cost for the transfusion services that purchase the units. The transfusion service must do additional testing and provide additional transport and storage, with more indirect costs for environmental controls, information systems, equipment maintenance, and professional staff to do the testing, manage the inventory, and meet regulatory expectations. Stokes et al. did a cost analysis at two hospitals in the United Kingdom (UK) and reported that the laboratory costs for providing transfusions were ~$28 (US dollars) per unit transfused (in 2015 dollars), with little variability depending on the different types of components or the number of units issued (8).
In contrast to what Stokes et al. reported, the cost of pre-transfusion testing can vary markedly depending on patient-specific factors, such as pre-existing antibodies and drug therapy. Antibodies need to be identified and units of blood compatible with those antibodies need to be found. Mazonson et al. found that the average cost per patient varied based on patient diagnosis, with obstetric patients having an average pre-transfusion testing cost of $69 and patients with warm autoantibodies having an average cost of $505 per transfusion event (9). Their data was collected between 2009 and 2011 so the associated costs would be expected to be higher today when adjusted for inflation (10). Hofmann et al. observed that 42.2% of inpatients had type and screen testing done (33.1% of medical inpatients versus 64.3% of surgical inpatients), even though only 6.3% of inpatients received any type of blood component (11). Cost accounting for all of those type and screen tests that were not associated with transfusions (35.9% of tests in Hofmann’s study) are not part of the calculation for the overall cost of transfusion. If they were, the indirect costs would be higher. The transfusion service laboratory that receives the unit of packed red cells from the blood center for $192 might add on another $58 for their direct and indirect costs for a total so far of $250.
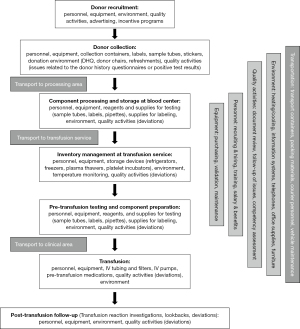
Once a unit of blood is ready to be transfused, there are direct costs for intravenous (IV) catheters, infusion sets and filters, and saline or other compatible fluid to prime the infusion set. The patient might require pre-medication to avoid a transfusion reaction. Indirect costs again include environmental controls, information systems, equipment maintenance (e.g., IV pumps and blood warmers), and nursing staff time to administer the transfusion, monitor the patient for a potential reaction, and meet regulatory expectations (12). Other indirect costs that might be added would be the cost of printing instructions for outpatients who receive transfusions or the cost of transfusion reaction investigations. In the study by Stokes et al., the per-unit nursing costs for providing transfusions were higher for the initial transfusion ($25.64) than for subsequent transfusions ($4.58) using 2015 dollars (8). Stokes et al., therefore, found that the cost of transfusion was $53 in addition to the acquisition cost of the component transfused. Most studies looking at the cost of transfusion have primarily looked at RBC transfusions (13-17). For the hypothetical transfusion listed above, these administrative direct and indirect costs might add another $35 to the $250 already described, for a total transfusion cost of $285. A summary of the mean costs reported for RBC transfusions from various countries is presented in Table 4. Variability in the costs listed might be due to factors such as whether or not RBC units are typically leukoreduced, the mean wages for staff who participate in transfusion activities, and differences in costs for meeting regulatory expectations (e.g., cost of testing or additional documentation requirements). Figure 1 outlines the flow of blood discussed in this section from collection to transfusion.
Table 4
| Author, reference | Location | Total cost of RBC transfusions† |
|---|---|---|
| Shander et al. (10) | EHMC, United States | $1,183.32 (€1,088.65) |
| RIH, United States | $726.05 (€667.97) | |
| CHUV, Switzerland | $611.44 (€562.52) | |
| AKH, Austria | $522.45 (€480.65) | |
| Toner et al. (12) | United States | $343.63 (€316.14) |
| Abraham & Sun (13) | Western Europe | €877.69 ($954.01) for 2 RBC units |
| Fragoulakis et al. (14) | Greece | €165.49 ($179.88) |
| Kleinerüschkamp et al. (15) | Germany | €147.43 ($160.25) |
| Mafirakureva et al. (16) | Zimbabwe | $130.94 (€120.46) |
| Rigal et al. (17) | France | €339.67 ($369.21) |
Values are those originally reported, not adjusted for inflation. †, first number is the value reported in the original article. The number in parenthesis is based on an exchange rate of $1.00=€0.92. RBCs, red blood cells; EHMC, Englewood Hospital Medical Center; RIH, Rhode Island Hospital; CHUV, Centre Hospitalier Universitaire Vaudois; AKH, General Hospital Linz.
Reimbursement for transfusions
In the US, reimbursement for blood and transfusion services can vary depending on whether the reimbursement comes from the federal government [Centers for Medicare/Medicaid Services (CMS)] or private insurers. Many private insurers use the same general payment schedule as CMS and variations to that are beyond the scope of this article so only CMS reimbursement will be discussed.
A couple of concepts common to all forms of reimbursement are that there must be documentation in the patient’s medical record to indicate what services (i.e., testing) and materials (i.e., units of blood) were provided and those services and materials must be considered medically necessary. Although it is acceptable to bill for services provided even when a transfusion does not occur (e.g., billing for type and screen testing), units of blood that were ordered, but not transfused cannot be billed to the patient. Similarly, if a patient needs special processing for their blood (e.g., antigen typing because they have red cell antibodies), the cost of that additional service (antigen testing) can be added to the patient’s bill. However, if a unit that had special antigen typing done is given to a patient who had no documented need for such typing, the cost of that additional typing cannot be billed to the patient (18).
Facilities use Health Care Common Procedure Coding system (HCPCS) codes and Current Procedural Terminology (CPT) codes for the outpatient setting. Specifically, HCPCS p-codes cover the blood components. The most commonly billed HCPCS p-code for blood is P9016, which is for leukoreduced RBC. Note that a p-code may be used every time a component is transfused. For a patient who receives two units of leukoreduced RBCs during an outpatient visit, the billing documentation should include two P9016 codes (or P9016 × 2), one for each unit. CPT codes are similar to ICD-10-PCS codes in that they are used for procedures, but are specifically for the outpatient setting instead of the inpatient setting. For a patient who receives a leukoreduced RBC unit during an outpatient visit, in addition to the p-code used for the actual unit of blood, the patient’s bill should include CPT code 36430 for the act of transfusing the blood (19,20).
Each year, CMS updates the list of codes that it uses as well as the amount it plans to pay for each code, which is published as the Hospital Outpatient Prospective Payment system (OPPS) final rule. For the patient who receives a unit of leukoreduced RBC, the 2022 Calendar Year OPPS lists reimbursement of $192.39 for the P9016 code and $405.37 for the 36430 code, or a total of $597.76 (19,20). As noted in Table 4, this amount would cover the acquisition cost for a unit of leukoreduced RBCs, but, as shown in Table 4, it might not adequately reimburse for the total cost of administering the transfusion.
Reimbursement for any hospital stay is predetermined based on a diagnosis related group (DRG). Based on previous amounts requested for reimbursement for a specific diagnostic category, CMS determines how much it is willing to pay. Similar to the OPPS, CMS publishes an annual report of how much it will pay in the Hospital Inpatient Prospective Payment System (IPPS) Final Rule. A study published by Jefferies et al. in 2001 presented an assessment of the relative cost of transfusion based on DRG done for 1995 admissions showed that transfusion accounted for as much as 12.7% of the DRG cost (DRG 473, acute leukemia without major operating room procedure) (21). Understandably, many DRGs have no associated transfusion costs, if those patients do not typically receive transfusions. No other studies specifically addressing this topic have been published. Since that study was done, the concepts of PBM have impacted transfusion trends, but there continues to be not much literature regarding their economic impacts.
Modalities of PBM
PBM exists in many ways and through different subspecialties in the hospital. Typically, the PBM program exists through a multidisciplinary committee in the hospital. It is there where different disciplines throughout the hospital (pathology, laboratory staff, nursing staff, physicians from different subspecialties) come together to discuss transfusion practice throughout the facility(ies). Guidelines and recommendations are discussed here as well as expected behaviors. Through facilities accredited by the Association for the Advancement of Blood and Biotherapies (AABB) or other professional associations, the medical director of the transfusion service has executive say over the transfusion policies in the facility (22).
Though the transfusion service medical director has the final say, it is best practice to meet with stakeholder physicians to discuss needs and wants to increase compliance and decrease costs associated with unnecessary transfusions. It is important to have this physician representation at PBM/transfusion practice committee meetings. Their participation ensures that physicians have input in the decisions being made by the transfusion service medical director, which should increase compliance throughout ordering providers. It also ensures they are aware of all changes that are being made, which include those being made to transfusion thresholds and feedback they would receive for orders that may not meet criteria. In addition to service behaviors, participation allows physicians to learn during meetings about transfusion practice in general and ask questions pertaining to transfusion
Evidence-based transfusion thresholds can be developed through the PBM program or transfusion practice committee. These thresholds are a guideline for providers for best practices regarding transfusion of blood and blood products. Developing these guidelines can prevent unnecessary transfusions in the hospital or limit transfusions to prevent multiple donor exposures. An example of this is providing minimal hemoglobin requirements for transfusion. Of note, it is important that although these guidelines represent best practice, they do not constitute a standard of care. Not every patient who meets these guidelines will require a transfusion. These circumstances are best discussed peer-to-peer with the ordering physician and transfusion service medical director.
Ordering behaviors, blood purchasing, blood utilization, and waste can and should all be monitored by the PBM program (23). These audits can show trends and deficits that can be addressed, leading to a better standard of care for patients. Ordering practices can be narrowed down to the physician and provider level to see if certain individuals need education on guidelines. Monitoring wastage in the transfusion service can help with modifying ordering of supplies, which includes consumables and testing supplies as well as blood components. This presents cost savings to the hospital since the hospital pays the blood center for components but may not be reimbursed if the components are wasted due to expiration or improper handling.
Since the advent of blood transfusions there have been known associated risks. More risks associated with transfusion have been identified throughout the years with newer technology being available to study in-vivo effects. Though these risks have been identified, risks such as infectious disease transmission have significantly decreased with more sensitive and specific screening assays and pre-donation questionnaires. Overall risks include transfusion-transmitted infectious disease (more are being discovered and can be associated with specific regions) as well as transfusion reactions (e.g., hemolytic transfusion reactions, non-hemolytic febrile transfusion reactions, transfusion related acute lung injury). The increase in discovered transmitted infectious diseases has led to more testing of blood components, as well as the discovery of immunologic complications associated with certain transfusions such as antibodies to human leukocyte antigens. This has led to an increase in costs for blood components, which includes an increase in delivery costs and payment of medical services. Recent studies have even shown a correlation between blood component transfusion and morbidity and mortality (24,25). With the inherent risks associated with the transfusion of blood components, it is important to reduce the number of transfusions when alternative therapies are available.
Certain interventions can help change the behavior of physicians regarding transfusing blood. These interventions, although simple, can be very effective when applied to physicians and in a multidisciplinary committee. Interventions include audits with feedback, audits with approval or disapproval of ordering practices, transfusion forms with transfusion criteria posted, as well as provider education (26). Systemic reviews of these interventions showed that these behaviors led to either a decrease in components transfused (i.e., one unit rather than two), or a decrease of overall blood component utilization.
In addition to simple interventions on the side of the transfusion service, new laboratory and hospital information systems can help aid in the modification of physician behavior in real-time. These systems work by providing recent laboratory values during blood component ordering as well as warnings when orders do not meet criteria set forth by the transfusion service. Ikoma et al., has shown that these live warnings in the computerized physician order entry (CPOE) system alone help change transfusion behavior with physicians. Through the use of live alerts when ordering, the authors were able to reduce multi-unit transfusions by 8.6% and increase guidance-indicated red cell transfusion by 10.6% (27). The live alerts provide real-time clinical support to the ordering physician to help either support or reject their decision to transfuse based off best practice guidelines set forth by their transfusion practice committee.
Preoperative optimization of hemoglobin
Blood loss during surgical procedures is an expected event. For very invasive procedures, there is a higher risk of blood loss, and the quantity of blood loss is generally greater than small procedures (e.g., cardiovascular surgeries and certain orthopedic procedures). Anemia, or a decrease in RBCs and/or hemoglobin concentration, is found in about 5% of the general population. This number increases with certain disease states and conditions (e.g., colorectal cancer patients ~46% and elderly orthopedic patients ~75%), which contributes to the population who are more likely to have highly invasive procedures (28). Identifying anemia preoperatively allows the surgeon to identify the origin of the patient’s anemia, as well as optimize the patient prior to surgery to improve post-surgical outcome.
Preoperative anemia contributes to post-operative anemia, which can result in a patient becoming transfusion dependent. Blood transfusions have associated risks, which include transfusion-transmitted disease, ischemic complications, and death. Patients who are sicker are more at-risk for preoperative anemia, post-operative anemia, and transfusion. These patients are also most at risk for transfusion-related complications. Although studies have shown a link with post-operative transfusion and mortality, a restrictive transfusion approach did not show a significant difference in outcome when compared to a liberal approach to post-operative transfusion (28,29). Even with a restrictive approach to transfusions, there continued to be an increased risk for complications in patients who required transfusion.
Discovering anemia early is the key to optimizing the patient. There is little to be done if anemia is discovered in the patient 4–7 days prior to their procedure. To be able to investigate and initiate treatment, it is most beneficial if anemia is discovered at least 3 to 4 weeks prior to their procedure (28,29). Following the discovery of anemia in presurgical patients, investigational studies should be done to identify the source of anemia. Nutritional deficiencies should be corrected if identified (e.g., B12 or iron deficiency). Erythropoietic stimulating agents (ESAs) may be utilized to optimize anemia prior to their procedure.
Identifying iron deficiency anemia (IDA) is an important aspect of PBM as it can lead to decreased need for blood transfusions and associated costs. Iron is essential for the production of hemoglobin, the protein in RBCs that carries oxygen throughout the body. When iron levels are low, the body cannot produce enough hemoglobin, leading to anemia (30).
IDA is a common cause of anemia, and its prevalence is high in surgical and critically ill patients. Early identification and treatment of IDA is important to optimize hemoglobin levels before surgery or other medical procedures, and to reduce the risk of transfusion (30).
Iron replacement therapy is the primary treatment for IDA. Oral iron supplements are often used as first-line therapy, but may not be effective in patients with severe anemia or those who are unable to tolerate oral iron due to gastrointestinal side effects. In these cases, IV iron may be used. IV iron therapy has been shown to be safe and effective in correcting IDA in surgical and critically ill patients, with minimal adverse effects (30).
Several IV iron formulations are available, including iron sucrose, ferric carboxymaltose (FCM), iron dextran, and ferumoxytol. Dosing and administration may vary depending on the formulation used and the severity of anemia. It is important to note that IV iron therapy should be used judiciously, as excessive dosing can lead to iron overload and associated complications (30).
One study by Bisbe et al. evaluated the cost-effectiveness of IV FCM compared to standard care for the treatment of IDA in patients undergoing major surgery. The authors found that FCM was cost-effective, with a projected cost savings of €111 per patient (31).
A 2015 study by Rodgers et al. found that the use of iron sucrose to treat anemia in an outpatient setting yielded substantial savings for the hospital system. They reported $37,656 in cost savings within the study when the associated costs and reimbursement rates of iron sucrose treatments were compared to those of transfusion (32).
Identifying and treating IDA is an important component of PBM and can lead to decreased need for blood transfusions and associated costs. IV iron therapy is a safe and effective treatment option for patients with severe anemia or those who are unable to tolerate oral iron and may be cost-effective compared to standard care (32).
Presurgical testing of patients should be done as early as three to four weeks in non-emergent/urgent circumstances (28,29). If the patient is anemic, these results should be shared with the patient’s primary care provider to allow for adequate treatment and optimization of the patient for surgery. It is up to the surgeon to decide whether to proceed with the procedure or to delay the procedure until the anemia is corrected to an acceptable level. Correcting the patient’s preoperative anemia can facilitate a better post-operative outcome and lower costs.
Intraoperative salvage
Intraoperative RBC salvage is a technique that is used to recycle shed blood during certain surgeries and procedures. Shed blood is collected from the patient and is anticoagulated. The blood is sent to a reservoir where it is washed and then can be transfused back to the patient (33). This is a form of autologous blood transfusion, where the patient receives their own blood. This minimizes allogeneic transfusion and mitigates the risks associated with donor blood. Although it reduces the risks associated with allogeneic blood, there are associated risks with autologous cell salvage as well as contraindications.
Cell salvage is recommended when approximately 20% of the patient’s blood will be shed throughout the procedure. This can only be done during certain procedures due to contraindications such as procedures where there is contamination with stool, urine, fat, amniotic fluid, or bone chips (33). Cell salvage is typically performed for cardiac, orthopedic, neuro-, and vascular surgeries, and liver transplantations. Despite the contraindication of contamination with amniotic fluid, a study published by Liu et al., showed that autologous cell salvage is safe and effective during obstetrical hemorrhage, specifically during cesarean sections (34). They showed that contamination from amniotic fluid or fetal components did not pose a risk to the patient.
A major concern regarding intraoperative cell salvage is bacterial contamination. For this reason, operations where there is contamination, such as stool, are a contraindication for use. The concern is that bacteria from the salvaged blood would be transfused causing septicemia and other postoperative complications. During cardiac surgery and liver transplantations, approximately 30% and 9%, respectively, of blood salvaged contained bacteria (35,36). Though the fear of postoperative septicemia and infection exists, there is no data in literature that support this. Both skin flora contamination as well as fecal flora contamination in salvaged blood have not caused an increase in infection after intraoperative cell salvage (37).
Contraindications such as malignancy, sickle cell disease, and thalassemia are still under investigation (33). These conditions have theoretical complications such as red cell alteration and reduced oxygen-carrying capacity for sickled cells and decreased cell survival in thalassemia patients. Although these complications are postulated, there are documented cases of these patients receiving intraoperative cell salvage without major complication. The concern with malignancy is that cancer cells could be infused back into the patient and lead to metastatic disease. There are no reports, to date, to support this (37).
Cell salvage is a safe alternative to allogeneic blood transfusion that also has cost saving benefits. The initial purchase of cell salvaging machines requires an upfront cost, but they are inexpensive to operate and maintain. Quality control for the machines is also inexpensive to the operating facility and generally requires testing such as hematocrit, total protein, potassium and albumin levels on a facility defined number of collected products monthly. This technique can save the facility money on transfusion related expenses (i.e., pre-transfusion testing, blood product acquisition, blood product testing, and blood product delivery to the patient). It has been shown that returning one or more units to the patient leads to economic value. In addition to the economic value, salvaged blood lacks storage lesion effects seen in stored blood. Therefore, the RBCs may be of higher quality, comparatively (37).
Pre-operative/rapid anemia clinics
Pre-operative or rapid anemia clinics are a key component of PBM programs as they allow for early identification and management of preoperative anemia, which is a major risk factor for transfusion. Several studies have examined the cost-effectiveness of pre-operative anemia clinics, with most finding that they are cost-effective compared to standard care.
One study by Wan et al. evaluated the cost-effectiveness of a pre-operative anemia clinic in orthopedic surgery patients. The clinic included preoperative testing and iron therapy for patients with anemia. The authors found that the clinic was cost-effective, with a projected cost savings of 254 EUR (284.43 USD) per patient (38).
Another study by Muñoz et al. evaluated the cost-effectiveness of a rapid anemia clinic in colorectal surgery patients. The clinic included preoperative testing and iron therapy for patients with anemia. The authors found that the clinic was cost-effective, with a projected cost savings of €272 per patient (39).
Multi-modal large-scale PBM programs that include a combination of pre-operative anemia management, intraoperative blood conservation techniques, and postoperative anemia management have also been shown to be cost-effective. One study by So-Osman et al. evaluated the cost-effectiveness of a PBM program in hip and knee arthroplasty patients. The program included preoperative anemia management, intraoperative cell salvage, and postoperative anemia management. The authors found that the program was cost-effective, with a projected cost savings of €231 per patient (40).
It is important to note that the costs of implementing PBM programs may vary depending on the patient population and healthcare setting. However, these studies demonstrate that PBM programs, including pre-operative or rapid anemia clinics, can be cost-effective and result in significant cost savings.
Cost analyses
Cost analyses are an important aspect of evaluating the economics of PBM programs, as they can provide insight into the potential cost savings associated with implementing PBM strategies. Several studies have examined the costs and cost-effectiveness of various PBM modalities, including preoperative optimization, intraoperative blood conservation techniques, and postoperative anemia management.
One study by Ternström et al. elvuated the cost-effectiveness of a PBM program, which included preoperative anemia management and restrictuve transfusion thresholds. The authors found that with the reduction in transfusion, approximately $218,930 (€161,623) were saved in the cost of blood product over the period of 1 year (41).
Another study by Ferraris et al. examined the cost savings associated with implementing a blood conservation program in cardiac surgery patients. The program included measures such as minimizing phlebotomy, using cell salvage, and implementing a transfusion trigger of hemoglobin <7 g/dL. The authors found that the program resulted in a 21% reduction in transfusions and a cost savings of $2,932 per patient (42).
A systematic review by Shander et al. examined the cost-effectiveness of various PBM interventions. The authors found that interventions such as preoperative anemia management, intraoperative blood conservation techniques, and the use of erythropoiesis-stimulating agents were cost-effective, with a projected cost savings of up to $1,367 per patient (43).
These studies demonstrate the potential cost savings associated with implementing PBM programs. However, it is important to note that the cost-effectiveness of PBM interventions may vary depending on the patient population and healthcare setting. Further research is needed to fully evaluate the economic impact of PBM programs and identify the most cost-effective strategies for various populations and settings. The modalities listed above can be used to help modify transfusion behavior to a more restrictive approach. Mitigating unnecessary transfusions can prevent associated cost, providing cost-savings to both the transfusion service and the patient.
Alternative therapies
Tranexamic acid (TXA) is a lysine analog that blocks the conversion of plasminogen to plasmin (44). This reduces the ability of plasminogen to bind fibrin, inhibiting fibrinolysis, and allowing fibrin clots to remain stable to sustain hemostasis. TXA is used topically, orally, and intravenously. Its use is recommended for patients with underlying disorders that cause hyperfibrinolysis and has been widely studied and recommended for improving outcomes and treating acute hemorrhage caused by trauma and postpartum bleeding, as demonstrated in the CRASH-22, MATTERS3, and WOMAN4 studies (45-47). TXA is widely used during surgeries that carry significant bleeding risk (48). Studies have demonstrated its efficacy in reducing blood loss, minimizing the need for transfusion, and improving outcomes whether it is used topically, orally, or intravenously during surgery (48-50).
An analysis by the United Kingdom National Clinical Guideline Centre found TXA to be equivalent or superior to all other interventions for reducing perioperative blood loss (50). In surgical patients that had a moderate to high risk of bleeding, they found that TXA was the most cost-effective option when compared to a placebo or usual care.
One study found that the use of TXA in total hip arthroplasty produced a direct cost savings of €47 ($50.55 USD) per patient (50), while also reducing potential downstream costs such as transfusion reactions, transfusion-transmitted infections, and prolonged hospital stays.
A cost-benefit analysis of TXA and blood transfusion for elective lumbar spine surgeries demonstrated there was a significant net cost savings of $328.69 (USD) per patient in long-length constructs when TXA was used (45). This study included both direct acquisition costs of packed red blood cells for their facility ($200) as well as indirect costs (type and screen, crossmatch, cold storage, and transportation), all of which bring the cost to approximately $800 per unit. Though statistically insignificant, there was still a net cost savings of $49.58 in the short-length constructs group.
Given the many ways TXA can be utilized, it is difficult to determine its economic value in every possible scenario, as cost and efficacy are dependent on dose and modality, but there is plenty of data to support it as a cost-effective option for reducing the need for transfusion.
Iron is an essential dietary mineral and necessary for hemoglobin production. Iron deficiency can be caused by an inadequate iron intake or functional defects that block the release of stored iron from cells (51). Regardless of the pathology, a lack of available iron over time can cause IDA. Both oral iron supplements and IV iron are used to treat IDA, though oral iron requires a greater amount of time to see benefits and is dependent on patient compliance that may be negatively impacted by side effects. IV iron has a more immediate benefit and is generally well tolerated. IV iron supplements are formulated as iron carbohydrate complexes. In the body, cells separate iron from its attached carbohydrate, and it is brought to the liver, spleen, and bone marrow by transferrin (52). There are many IV iron formulations; a commonly used one is FCM. FCM has been shown to reduce the need for allogeneic blood transfusion perioperatively and may provide a prolonged beneficial effect on hemoglobin level in patients with IDA (51).
ESAs are recombinant erythropoietin drugs that stimulate the bone marrow to make RBCs (53). They are used for the treatment of anemia that is caused by chronic kidney disease, chemotherapy, and the human immunodeficiency virus treatment drug zidovudine (54). They are also being used to promote independence or a lesser dependence of RBC transfusion in patients with myelodysplastic syndrome (55). Clinical trials on ESAs have demonstrated that risks associated with full correction of anemia outweigh the benefits, so it may be better used for partial correction in order to mitigate this risk (56).
Associated costs
Alternative therapies that can be taken orally (e.g., iron) are most cost-effective because they require no additional materials, labor, or clinical space. Unfortunately, they have a more limited clinical utility and depend on patient compliance. Costs associated with therapies that must be given intravenously include drug cost, nursing time, clinic space, administrator time, and disposable materials needed for the preparation and infusion of the therapy (50). Disposable materials needed may include cannulas, needles, syringes, dressings, IV sets and sodium chloride solution. Exact needs depend on the therapy, route of delivery, and specific formulations used. All alternative therapies covered are stored at room temperature, except for ESAs, which require refrigeration.
When compared to the transfusion of blood and blood products, these alternative therapies do carry a lesser cost. This is partially due to the cost associated with the acquisition, manufacturing, distribution, and testing associated with blood and blood products. In addition to the lower cost, these therapies have considerably less risk. Blood and blood products come from donors and have inherent risks associated such as immunological complications and infectious disease contamination.
Common clinical practice vs. alternative
In a study from John Hopkins in 2016, it was shown that a decrease in transfusion orders (23.9% to 17.1%, P<0.001) and utilization (12%, P<0.035) showed a marked decrease in cost (56). The decrease in transfusions and utilization were achieved mainly by the implementation of evidence-based transfusion guidelines, clinical decision support in the CPOE, and audits providing peer comparison for guideline compliance. The acquisition cost avoidance was $181,887/year and $582,039–$873,058/year for activity-based cost (57). The majority of this, approximately 93%, was attributed to a decrease in red cell transfusions alone.
With new data emerging in transfusion medicine with regard to transfusion dosing, single unit transfusions are becoming more of the standard of practice in the U.S. The old mentality for dosing RBCs was that, if you are going to give one unit, you might as well give two. This led to an increase in blood utilization and an increase to donor exposure in patients where a single unit may have sufficed. In addition, decreasing the amount of blood transfused has the potential for substantial cost savings. Warner et al. showed that implementing a single-unit default for electronic ordering led to a decrease in RBC units transfused (58). They saw a 13.9% decrease in transfusion (32,528 to 27,497, P<0.0001) through the 2-year observational study, which lead to a reduction in transfusion related costs of about $4 million.
Lastly, cost savings can be seen with proper inventory management and wastage prevention strategies. The acquisition and required testing of blood and blood products cost hundreds of dollars, which is charged to the patient. When these products are wasted, the hospital or transfusion service are not reimbursed for the associated costs. Implementing simple techniques, including increased education, improved component transport apparatus, and print and digital messaging to staff, can decrease wastage. Collins et al. showed that even a small decrease in wastage (RBC 0.67–0.56%, P<0.001; platelets 3.71–2.81%, P<0.001; plasma 1.14–1.40%, P<0.001) presented a cost decrease of $131,520, which excludes any cost of interventions (23).
Conclusions
The cost of transfusion, which includes manufacturing, acquisition, and distribution, carries a large price tag in the U.S. When compared to alternative therapies, there is potential for cost savings for both the transfusion service and the patient. In addition to the costs savings, patients may have better surgical outcomes when using alternative therapies.
Acknowledgments
We would like to thank Dr. Richard Gammon (OneBlood, USA) for his work as the guest editor on this manuscript. He provided insight and expertise that was greatly appreciated.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Richard Gammon) for the series “Patient Blood Management’s Role in Current Healthcare Environment” published in Annals of Blood. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aob.amegroups.com/article/view/10.21037/aob-22-35/rc
Peer Review File: Available at https://aob.amegroups.com/article/view/10.21037/aob-22-35/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-22-35/coif). The series “Patient Blood Management’s Role in Current Healthcare Environment” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Franchini M, Marano G, Veropalumbo E, et al. Patient Blood Management: a revolutionary approach to transfusion medicine. Blood Transfus 2019;17:191-5. [PubMed]
- Bolcato M, Russo M, Trentino K, et al. Patient blood management: The best approach to transfusion medicine risk management. Transfus Apher Sci 2020;59:102779. [Crossref] [PubMed]
- Blood Components. 2022. Accessed August 3, 2022. Available online: https://www.redcrossblood.org/donate-blood/how-to-donate/types-of-blood-donations/blood-components.html
- Harmening DM. Modern Blood Banking & Transfusion Practices. 6th edition. Philadelphia, PA, USA: F.A. Davis Company; 2012:312-8.
- The cost of blood: multidisciplinary consensus conference for a standard methodology. Transfus Med Rev 2005;19:66-78. [Crossref] [PubMed]
- Stubbs JR. Wrapping our arms around the cost of transfusion therapy. Transfusion 2014;54:259-62. [Crossref] [PubMed]
- Mowla SJ, Sapiano MRP, Jones JM, et al. Supplemental findings of the 2019 National Blood Collection and Utilization Survey. Transfusion 2021;61:S11-35. [Crossref] [PubMed]
- Stokes EA, Wordsworth S, Staves J, et al. Accurate costs of blood transfusion: a microcosting of administering blood products in the United Kingdom National Health Service. Transfusion 2018;58:846-53. [Crossref] [PubMed]
- Mazonson P, Efrusy M, Santas C, et al. The HI-STAR study: resource utilization and costs associated with serologic testing for antibody-positive patients at four United States medical centers. Transfusion 2014;54:271-7. [Crossref] [PubMed]
- Shander A, Hofmann A, Ozawa S, et al. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion 2010;50:753-65. [Crossref] [PubMed]
- Hofmann A, Ozawa S, Shander A. Activity-based cost of platelet transfusions in medical and surgical inpatients at a US hospital. Vox Sang 2021;116:998-1004. [Crossref] [PubMed]
- Toner RW, Pizzi L, Leas B, et al. Costs to hospitals of acquiring and processing blood in the US: a survey of hospital-based blood banks and transfusion services. Appl Health Econ Health Policy 2011;9:29-37. [Crossref] [PubMed]
- Abraham I, Sun D. The cost of blood transfusion in Western Europe as estimated from six studies. Transfusion 2012;52:1983-8. [Crossref] [PubMed]
- Fragoulakis V, Stamoulis K, Grouzi E, et al. The cost of blood collection in Greece: an economic analysis. Clin Ther 2014;36:1028-1036.e5. [Crossref] [PubMed]
- Kleinerüschkamp AG, Zacharowski K, Ettwein C, et al. Cost analysis of patient blood management. Anaesthesist 2016;65:438-48. [PubMed]
- Mafirakureva N, Nyoni H, Nkomo SZ, et al. The costs of producing a unit of blood in Zimbabwe. Transfusion 2016;56:628-36. [Crossref] [PubMed]
- Rigal JC, Riche VP, Tching-Sin M, et al. Cost of red blood cell transfusion; evaluation in a French academic hospital. Transfus Clin Biol 2020;27:222-8. [Crossref] [PubMed]
- Association for the Advancement of Blood and Biotherapies. AABB billing guide for blood products and related services. Bethesda, MD, USA: Association for the Advancement of Blood & Biotherapies; 2020. Accessed August 31, 2022. Available online: https://www.aabb.org/news-resources/resources/billing-guide
- Centers for Medicare and Medicaid Services. Medicare Program: Hospital Outpatient Prospective Payment and Ambulatory Surgical Center Payment Systems and Quality Reporting Programs; Price Transparency of Hospital Standard Charges; Radiation Oncology Model. Accessed August 31, 2022. Available online: https://public-inspection.federalregister.gov/2021-24011.pdf
- Centers for Medicare and Medicaid Services. Fact Sheet. CY 2022 Medicare Hospital Outpatient Prospective Payment System and Ambulatory Surgical Center Payment System Final Rule (CMS-1753FC). Accessed August 31, 2022. Available online: https://www.cms.gov/newsroom/fact-sheets/cy-2022-medicare-hospital-outpatient-prospective-payment-system-and-ambulatory-surgical-center-0
- Jefferies LC, Sachais BS, Young DS. Blood transfusion costs by diagnosis-related groups in 60 university hospitals in 1995. Transfusion 2001;41:522-9. [Crossref] [PubMed]
- Standards for Blood Banks and Transfusion Services. 33rd edition. Bethesda, MD, USA: Association for the Advancement of Blood & Biotherapies Press; 2022.
- Collins RA, Wisniewski MK, Waters JH, et al. Effectiveness of multiple initiatives to reduce blood component wastage. Am J Clin Pathol 2015;143:329-35. [Crossref] [PubMed]
- Paone G, Likosky DS, Brewer R, et al. Transfusion of 1 and 2 units of red blood cells is associated with increased morbidity and mortality. Ann Thorac Surg 2014;97:87-93; discussion 93-4. [Crossref] [PubMed]
- Kansagra AJ, Stefan MS. Preoperative Anemia: Evaluation and Treatment. Anesthesiol Clin 2016;34:127-41. [Crossref] [PubMed]
- Tinmouth A, Macdougall L, Fergusson D, et al. Reducing the amount of blood transfused: a systematic review of behavioral interventions to change physicians' transfusion practices. Arch Intern Med 2005;165:845-52. [Crossref] [PubMed]
- Ikoma S, Furukawa M, Busuttil A, et al. Optimizing Inpatient Blood Utilization Using Real-Time Clinical Decision Support. Appl Clin Inform 2021;12:49-56. [Crossref] [PubMed]
- Smith A, Moon T, Pak T, et al. Preoperative Anemia Treatment With Intravenous Iron in Patients Undergoing Major Orthopedic Surgery: A Systematic Review. Geriatr Orthop Surg Rehabil 2020;11:2151459320935094. [Crossref] [PubMed]
- Greenberg JA, Zwiep TM, Sadek J, et al. Clinical practice guideline: evidence, recommendations and algorithm for the preoperative optimization of anemia, hyperglycemia and smoking. Can J Surg 2021;64:E491-509. [Crossref] [PubMed]
- Cappellini MD, Musallam KM, Taher AT. Iron deficiency anaemia revisited. J Intern Med 2020;287:153-70. [Crossref] [PubMed]
- Bisbe E, García-Erce JA, Díez-Lobo AI, et al. A multicentre comparative study on the efficacy of intravenous ferric carboxymaltose and iron sucrose for correcting preoperative anaemia in patients undergoing major elective surgery. Br J Anaesth 2011;107:477-8. Erratum in: Br J Anaesth 2015;115:154. [Crossref] [PubMed]
- Rodgers W, Amrane S, Tsai T, et al. Intravenous Iron Infusion as a Cost-Effective Alternative to Transfusion of Packed Red Blood Cells Obstetrics & Gynecology 2015;125:46S. [128]. [Crossref]
- Esper SA, Waters JH. Intra-operative cell salvage: a fresh look at the indications and contraindications. Blood Transfus 2011;9:139-47. [PubMed]
- Liu Y, Li X, Che X, et al. Intraoperative cell salvage for obstetrics: a prospective randomized controlled clinical trial. BMC Pregnancy Childbirth 2020;20:452. [Crossref] [PubMed]
- Bland LA, Villarino ME, Arduino MJ, et al. Bacteriologic and endotoxin analysis of salvaged blood used in autologous transfusions during cardiac operations. J Thorac Cardiovasc Surg 1992;103:582-8. [Crossref] [PubMed]
- Kang Y, Aggarwal S, Pasculle AW, et al. Bacteriologic study of autotransfusion during liver transplantation. Transplant Proc 1989;21:3538. [PubMed]
- Frank SM, Sikorski RA, Konig G, et al. Clinical Utility of Autologous Salvaged Blood: a Review. J Gastrointest Surg 2020;24:464-72. [Crossref] [PubMed]
- Wan S, Sparring V, Cabrales DA, et al. Clinical and Budget Impact of Treating Preoperative Anemia in Major Orthopedic Surgery-A Retrospective Observational Study. J Arthroplasty 2020;35:3084-8. [Crossref] [PubMed]
- Muñoz M, Acheson AG, Auerbach M, et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2017;72:233-47. [Crossref] [PubMed]
- So-Osman C, Nelissen RG, Koopman-van Gemert AW, et al. Patient blood management in elective total hip- and knee-replacement surgery (Part 1): a randomized controlled trial on erythropoietin and blood salvage as transfusion alternatives using a restrictive transfusion policy in erythropoietin-eligible patients. Anesthesiology 2014;120:839-51. [Crossref] [PubMed]
- Ternström L, Hyllner M, Backlund E, et al. A structured blood conservation programme reduces transfusions and costs in cardiac surgery. Interact Cardiovasc Thorac Surg 2014;19:788-94. [Crossref] [PubMed]
- Society of Thoracic Surgeons Blood Conservation Guideline Task Force. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg 2007;83:S27-86. [Crossref] [PubMed]
- Shander A, Fink A, Javidroozi M, et al. Appropriateness of allogeneic red blood cell transfusion: the international consensus conference on transfusion outcomes. Transfus Med Rev 2011;25:232-246.e53. [Crossref] [PubMed]
- Ehresman J, Pennington Z, Schilling A, et al. Cost-benefit analysis of tranexamic acid and blood transfusion in elective lumbar spine surgery for degenerative pathologies. J Neurosurg Spine 2020; Epub ahead of print. [Crossref] [PubMed]
- Roberts I, Shakur H, Coats T, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess 2013;17:1-79. [Crossref] [PubMed]
- Morrison JJ, Dubose JJ, Rasmussen TE, et al. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Arch Surg 2012;147:113-9. [Crossref] [PubMed]
- Shakur H, Elbourne D, Gülmezoglu M, et al. The WOMAN Trial (World Maternal Antifibrinolytic Trial): tranexamic acid for the treatment of postpartum haemorrhage: an international randomised, double blind placebo controlled trial. Trials 2010;11:40. [Crossref] [PubMed]
- Haratian A, Shelby T, Hasan LK, et al. Utilization of Tranexamic Acid in Surgical Orthopaedic Practice: Indications and Current Considerations. Orthop Res Rev 2021;13:187-99. [Crossref] [PubMed]
- Ockerman A, Vanassche T, Garip M, et al. Tranexamic acid for the prevention and treatment of bleeding in surgery, trauma and bleeding disorders: a narrative review. Thromb J 2021;19:54. [Crossref] [PubMed]
- Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop 2005;76:314-9. [Crossref] [PubMed]
- Ionescu A, Sharma A, Kundnani NR, et al. Intravenous iron infusion as an alternative to minimize blood transfusion in peri-operative patients. Sci Rep 2020;10:18403. [Crossref] [PubMed]
- Silverstein SB, Rodgers GM. Parenteral iron therapy options. Am J Hematol 2004;76:74-8. [Crossref] [PubMed]
- Erythropoiesis-Stimulating Agents (ESA). U.S. Food and Drug Administration. 2017. Accessed July 25, 2022. Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-erythropoiesis-stimulating-agents-esa-epoetin-alfa-marketed-procrit-epogen-darbepoetin
- EPOGEN Package Insert. Amgen Inc. 2018. Accessed July 25, 2022. Available online: https://www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/Epogen/epogen_pi_hcp_english.pdf
- AJMC Peer Exchange. Considering The Role Of Esas And Blood Transfusions In MDS Management. 2022. Accessed July 25, 2022. Available online: https://www.ajmc.com/view/considering-the-role-of-esas-and-blood-transfusions-in-mds-management
- Drüeke TB. Lessons from clinical trials with erythropoiesis-stimulating agents (ESAs). Renal Replacement Therapy 2018;4:46. [Crossref]
- Thakkar RN, Lee KH, Ness PM, et al. Relative impact of a patient blood management program on utilization of all three major blood components. Transfusion 2016;56:2212-20. [Crossref] [PubMed]
- Warner MA, Schaefer KK, Madde N, et al. Improvements in red blood cell transfusion utilization following implementation of a single-unit default for electronic ordering. Transfusion 2019;59:2218-22. [Crossref] [PubMed]
Cite this article as: Nalezinski SR, Berg M, Labrecque C. Economics of patient blood management in the United States: a narrative review. Ann Blood 2024;9:6.