
Predictive value of the lactate dehydrogenase-to-albumin ratio (LAR) in classical Hodgkin’s lymphoma
Highlight box
Key findings
• The lactate dehydrogenase-to-albumin ratio (LAR) might be a potential biomarker for predicting overall survival (OS) in patients with classical Hodgkin’s lymphoma (cHL).
What is known and what is new?
• There is a strong interest in identifying simple, highly-sensitive, low-cost, and easily obtainable laboratory assessments that improve the fast prognosis of cHL. LAR has been demonstrated to be prognostic of OS in cancer.
• LAR >12.5 (hazard ratio =10.524, P<0.05) is an independent prognostic factor in cHL patients. Patients with LAR ≤12.5 had a better 3-year OS compared with patients with LAR >12.5 (93.4% vs. 0%; P<0.0001).
What is the implication, and what should change now?
• LAR may be a good independent prognostic indicator for OS in cHL patients. A multi-center prospective study to validate the LAR for predicting worsening in patients with cHL is the next step.
Introduction
Classical Hodgkin’s lymphoma (cHL) is a B-cell neoplasm with an incidence of ~3/100,000 and it accounts for ~15% of all malignant lymphomas affecting young adults (1). The lack of specific and sensitive non-invasive diagnostic tests remains a significant obstacle to long term monitoring and surveillance of patients with cHL. In this sense, there is a strong interest in identifying simple, highly-sensitive, low-cost, and easily obtainable laboratory assessments that improve or help the fast prognosis of cHL. cHL progression and prognosis are complex, with many contributing factors such as the immune response, nutritional status, and cancer-related inflammation (2). Recently, lactate dehydrogenase (LDH)-to-albumin ratio (LAR) has been demonstrated to be prognostic of overall survival (OS) in patients with colon cancer, esophageal cancer, colorectal carcinoma, and pancreatic cancer, with optimal cut-off values of 4.91, 5.5, 5.27, and 6.5, respectively (3-6). A 5-year OS of 13.3% was related to patients with LAR >5.5 (5). On the other hand, a 2.5-year OS of 55.2% in the group with high LAR (>5.27) was determined (4). The 3- and 5-year OS were 56% and 44% in the high LAR group (>4.91), respectively, was reported by Xie et al. (3). Hence, the study’s goal was to evaluate whether LAR could predict the OS of patients with cHL. We present this article in accordance with the REMARK reporting checklist (available at https://aob.amegroups.com/article/view/10.21037/aob-23-24/rc).
Methods
This was a single-center retrospective study performed at a tertiary hospital in Mexico (UMAE Hospital de Especialidades No. 14, Centro Médico Nacional “Adolfo Ruiz Cortines”). Data of 44 patients with newly diagnosed and untreated cHL between January 2015 and January 2022 were evaluated. OS was defined as the period from diagnosis until the date of last follow-up or death due to any cause. Follow-up data of the patients were obtained using the electronic medical records from hospital, follow-up visits, or by phone call. The last follow-up date was until 20 January 2022, and patients who were still alive at the end of the follow-up or in case of loss to follow-up were censored for OS analysis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethical and research committee from the UMAE H. E. No. 14, Mexican Institute of Social Security (IMSS) (Registration number: R-2021-3001-103). Informed consent was waived given the retrospective nature of the research. The patients included in this study were: (I) patients with age of 16 years of age or older; (II) patients with no receipt of previous therapy; (III) patients diagnosed with cHL using histopathological and immunohistochemical studies; (IV) patients with samples analyzed using a panel of antibodies specific for CD15 (Lewis X antigen) and CD30 (Ki-1 antigen), which are necessary to provide a confident diagnosis. In addition, the patients were eligible for the study if they had performed a positron emission tomography/computed tomography (PET/CT) according to local protocols and scanner procedures. Patients were stratified into early-stage (I–II) and advanced-stage (III–IV) cHL according to the Ann Arbor staging system.
The patients with early-stage cHL were treated with six cycles of ABVD [doxorubicin (adriamycin), bleomycin, vinblastine, and dacarbazine] and rituximab-ABVD. Patients with advanced-stage cHL were treated with six to eight cycles of BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone), LOPP-ABV (lomustine, vincristine, procarbazine, prednisone), rituximab-BEACOPP or escalated BEACOPP (eBEACOPP) regimens. Rituximab combined with chemotherapy was the first-line induction therapy of CD20 positive cHL.
Baseline total platelets, lymphocytes, neutrophil counts, and serum albumin and LDH levels were routinely measured when cHL was diagnosed. The definitions of the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), platelet-to-albumin ratio (PAR), systemic immune-inflammation index (SII), prognostic nutritional index (PNI), LAR, LDH-to-lymphocyte ratio (LLR) and neutrophil-to-albumin ratio (NAR) were calculated as follows: NLR = absolute neutrophil count/absolute lymphocyte count; PLR = platelet counts/absolute lymphocyte count; PAR = platelet counts/serum albumin level (g/L); SII = platelets counts × absolute neutrophil count/absolute lymphocyte count; PNI = serum albumin level (g/L) + 5 × absolute lymphocyte count; LAR = serum LDH level (U/L)/albumin level (g/L); LLR = serum LDH level (U/L)/absolute lymphocyte count; NAR = absolute neutrophil count/serum albumin level (g/L).
Statistical analysis
Categorical variables were represented as numbers, frequencies, or percentages (%). The data distribution was analyzed with the Shapiro-Wilks test, histogram, and Q-Q plots. Continuous variables with normal distribution were presented as mean (± standard deviation), and the differences between groups were analyzed with Student’s t-test. Continuous variables with nonparametric distribution were expressed as the median and interquartile range (IQR), and the difference between groups was analyzed using the Wilcoxon rank sum test. The association between the early- and advanced-Ann Arbor stages groups and clinical-demographic characteristics was performed with Fisher’s exact test (two-sided). The correlation between biomarker levels and Ann Arbor stages was calculated using the Spearman correlation test. Receiver operating characteristic (ROC) curve and area under the curve (AUC) were used to determine the optimal cut-off value. Patients were dichotomized into less than and greater than the cut-off values. Survival curves for OS were plotted via the Kaplan-Meier method, and the difference between patients’ subgroups was compared using the log-rank test. The Cox proportional hazard model was used to evaluate each variable’s prognostic value. The results were reported as hazard ratio (HR) and 95% confidence interval (CI) and visualized as a forest plot. A P value <0.05 was considered a significant difference. P values were adjusted using the Bonferroni corrections (P corrected) to compensate for the effect of multiple hypothesis testing. Data analysis was performed using MedCalc and Rstudio software (version 4.03; R Foundation for Statistical Computing, Vienna, Austria).
Results
The forty-four patients (26 females and 18 males) were classified using the Ann Arbor staining system as early-stage (stage I/II) and advanced-stage (stage III/IV) cHL. Most patients had advanced-stage cHL (26 cases, 59.1%). Both groups’ mean age was 34 years old (IQR, 27–43 years old) (P=1). The predominant histology was nodular sclerosis HL in 12 (66.7%) and 17 (65.4%) patients with early-stage and advanced-stage cHL, respectively. Regarding pretreatment blood test findings, patients with advanced-stage cHL, on average, had to have decreased neutrophils (means: 7.61×109/L vs. 7.30×109/L, P<0.0001), albumin (means: 41.11 vs. 34.92 g/L, P=0.002) and PNI (means: 49.39 vs. 41.76, P=0.001), and an increased LDH (medians: 165 vs. 255 U/L, P=0.03), and LAR (medians: 4.14 vs. 7.25, P<0.0005). Ann Arbor stages had a no significant correlation with neutrophils (r=0.0057, P=0.97) and these were negatively correlated with the albumin (r=−0.62, P<0.0001) and PNI (r=−0.69, P<0.0001). Whereas a positive correlation between Ann Arbor stages and LDH (r=0.57, P<0.0001), and LAR (r=0.74, P<0.0001) was observed in the analysis.
Univariate Cox regression analysis demonstrated that LLR >260.91 (HR =5.567, 95% CI: 1.217–25.459, P=0.0269), and LAR >12.5 (HR =23.693, 95% CI: 3.126–179.578, P=0.002) were significant prognostic factors for OS (Table 1). Although, the erythrocyte sedimentation rate (ESR) is a prognostic factor in Hodgkin’s lymphoma (HL), this had no prognostic importance in this population (HR =2.61, 95% CI: 0.56–12.07, P=0.219). Multivariate Cox regression analysis suggested that LAR >12.5 (HR =10.524, 95% CI: 1.125–98.457, P=0.039) is an independent prognostic factor in cHL patients for predicting OS (Table 1).
Table 1
| Variables | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Gender, male | 2.451 | 0.539–11.139 | 0.246 | – | – | – | |
| ECOG ≥2 | 2.882 | 0.632–13.148 | 0.172 | – | – | – | |
| IPS ≥2 | 3.448 | 0.414–28.703 | 0.252 | – | – | – | |
| Advanced-stage cHL | 2.811 | 0.526–15.026 | 0.227 | – | – | – | |
| EBV serology, positive | 0.995 | 0.990–0.999 | 0.998 | – | – | – | |
| B symptoms | 1.039 | 0.229–4.711 | 0.96 | – | – | – | |
| Organomegaly | 1.721 | 0.279–10.614 | 0.559 | – | – | – | |
| Bulky | 1.653 | 0.347–7.886 | 0.528 | – | – | – | |
| ESR | 2.61 | 0.56–12.07 | 0.219 | – | – | – | |
| NLR | 0.998 | 0.996–0.999 | 0.998 | – | – | – | |
| PLR | 0.219 | 0.041–1.177 | 0.076 | – | – | – | |
| SII | 0.789 | 0.152–4.088 | 0.777 | – | – | – | |
| LLR | 5.567 | 1.217–25.459 | 0.0269* | 3.345 | 0.552–20.254 | 0.188 | |
| PNI | 0.379 | 0.072–1.99 | 0.251 | – | – | – | |
| LAR | 23.693 | 3.126–179.578 | 0.002* | 10.524 | 1.125–98.457 | 0.039* | |
| PAR | 4.758 | 0.876–25.815 | 0.070 | – | – | – | |
| NAR | 0.808 | 0.155–4.204 | 0.8 | – | – | – | |
*, statistically significant differences (P<0.05). cHL, classical Hodgkin lymphoma; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Collaborative Oncology Group; IPS, International Prognostic Score; EBV, Epstein-Barr virus; ESR, erythrocyte sedimentation rate; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index; LLR, lactate dehydrogenase-to-lymphocyte ratio; PNI, prognostic nutritional index; LAR, lactate dehydrogenase-to-albumin ratio; PAR, platelet-to-albumin ratio; NAR, neutrophil-to-albumin ratio.
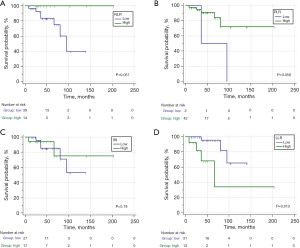
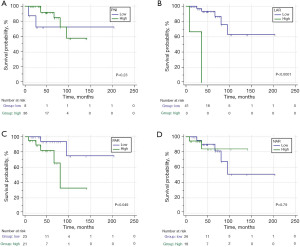
In order to evaluate the predictive value of the parameters involved in the OS, ROC curves were carried out to obtain the cut-off points of each variable. According to the appropriate cut-off point of NLR (≤6.86), PLR (≤125), SII (≤2183.86), LLR (>260.91), PNI (≤38.7), LAR (>12.5), PAR (>8.93), and NAR (≤0.21) in association with OS of cHL patients, the sample was divided into low and high values groups for the survival analysis (Figure 1 and Figure 2). Thirty-seven cHL patients relapsed or progressed in this study, and seven cHL patients died due to various causes. The median follow-up time was 47 months (range, 27.75–69.25 months). The Kaplan-Meier survival analysis demonstrated that higher LLR (Figure 1D), LAR (Figure 2B), and PAR (Figure 2C) were significantly correlated with shorter survival times. Compared to the patients with LLR >260.91, 3-year OS was higher in patients with LLR ≤260.91 (68.4% vs. 93.4%; P<0.05). Whereas the 3-year OS was higher in patients with PAR ≤8.93 that in the patients with PAR >8.93 (93.8% vs. 81.8%; P<0.05). Patients with LAR ≤12.5 had a better 3-year OS compared with patients with LAR >12.5 (93.4% vs. 0%; P<0.0001). The survival estimates were calculated using the log rank test.
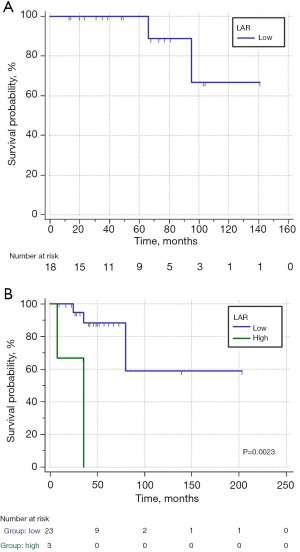
Furthermore, the cHL patients were divided into two groups according to the Ann Arbor stage to further verify the prognostic value of LAR. The patients with stages I/II did not have high LAR, and the 3-year OS was 100% in patients with low LAR (≤12.5) (Figure 3A). The 3-year OS was significantly shorter in patients with high LAR (>12.5) compared with patients with low LAR in stages III/IV (0% vs. 88.4%; P<0.005) (Figure 3B). The patients with high LAR and stage III/IV disease did not survive after 3 years of follow-up. Therefore, the trend of remarkable correlations to the worse OS by LAR >12.5 was consistent.
Discussion
There is interest in identifying simple, low-cost laboratory assessments that improve current risk and prognostic stratification approaches in cHL. For example, the use of serum calprotectin (S100A8/A9) and ferritin levels as prognostic markers in lymphoma has been suggested (7,8). Initially, this study analyzes for the first time the association between cHL stages and NLR, PLR, SII, LLR, PNI, LAR, PAR, and NAR, suggesting that LAR was strongly correlated with Ann Arbor stages. Furthermore, this study evaluated the prognostic role of NLR, PLR, SII, LLR, PNI, LAR, PAR, and NAR in patients with cHL. Zhao et al. and He et al. demonstrated that PLR, NLR, and PNI have prognostic value in the survival of patients with peripheral T-cell and diffuse large B cell lymphomas (9,10), PAR was reported as a predictive value for malignant neoplasms (11). On the other hand, pretreatment NLR, PLR, PNI, and SII have been described as progression factors in cHL (12-15). Interestingly, the results showed that only LAR was a strong predictor of survival outcomes with better performance than other well-known immune-inflammatory biomarkers in patients with cHL and other lymphomas. LLR was strongly related to poor survival in univariate analysis but not multivariate analysis. Among all biomarkers for lymphomas, LAR >12.5 was a good prognostic indicator for OS in cHL patients with an HR =10.524 (P<0.05), similar to pretreatment 18F-fluorodeoxyglucose (FDG) PET scans (HR =14.5, P=0.012) where a sample of 42 patients with newly diagnosed HL was used (16). Recently, LAR has been demonstrated to be prognostic of OS in patients with colon cancer, esophageal cancer, colorectal carcinoma, and pancreatic cancer, with optimal cut-off values of 4.91, 5.5, 5.27, and 6.5, respectively (3-6). In contrast, the optimal cut-off value of LAR obtained in this study was 12.5. Feng et al. reported a 5-year OS of 13.3% in patients with LAR >5.5 (5). In comparison, Aday et al. determined a 2.5-year OS of 55.2% in the group with high LAR (>5.27) (4). The 3- and 5-year OS were 56% and 44% in the high LAR group (>4.91), respectively, in the study performed by Xie et al. (3). In this study, the 3-year OS was 0% for patients with LAR >12.5. In this sense, there is no specific standard for the optimal cut-off LAR point, likely because of geographic regions, sample size, type of cancer, or screening criteria. Nevertheless, LAR was remarkably correlated to worse OS, including when the patients were stratified for advanced-stage cHL. The 3-year OS was significantly shorter in patients with high LAR than in patients with low LAR (0% vs. 88.4%; P<0.005). Mirili et al. reported that NLR, PNI, and SII are independent predictive factors for OS, where the median 5-year OS was shorter in patients with high NLR (≥4.3), low PNI (≤37.3), and high SII (≥1,628) (15). Moreover, Paydas et al. demonstrated that PNI ≤45.2 is a highest risk for OS in patients with cHL (14). In this regard, Simon et al. demonstrated that absolute lymphocyte-to-monocyte ratio has prognostic value in HL (17). However, NLR, PNI, and SII were not independent poor prognostic indicators for cHL in this study. Of note, the present study differs from these prior reports in that it focuses on the Mexican population and finds that LAR, rather than NLR, PLR, SII, LLR, PNI, PAR, and NAR, is an independent prognostic factor for OS in cHL patients. Together, these findings suggested that LAR should be a more objective marker that reflects the balance between host immune and inflammatory response than all the other systemic inflammation indexes such as PNI, NLR, PLR, and SII investigated in this study. Nevertheless, molecular mechanisms underlying the relationship between LAR and cHL are still unclear. Alcoceba et al. reported a high LDH and low serum albumin levels in patients with advanced-stage cHL (18). In this regard, by combing pretreatment albumin and LDH, LAR can simultaneously reflect nutritional status, tumor burden, and systemic inflammation, which may have a higher value in advanced-stage cHL predicting. A possible explanation for the close relationship between LAR and advanced-stage cHL could be that LDH and albumin can modulate the immune system’s inflammatory reaction due to their potential utility as circulating cancer biomarkers. Due to albumin and LDH being regulated by a different mechanism, LAR could reduce the influence of a single factor on the mechanism involved in the development of advanced-stage and prognostic of cHL. However, more evidence is needed to clarify the specific mechanism to advance our understanding.
This study had limitations. First, the small sample size is a weakness of our study. Nevertheless, for rare diseases such as cHL, it is hard to perform studies with larger sample sizes. Second, LAR only was evaluated before treatment, so the LAR values may significantly affect progression and prognosis throughout the treatment cycle. Third, our study’s log-rank test for the NLR, PLR, SII, PNI, and NAR was P>0.05; thus, the Kaplan-Meier curves are not statistically significantly different. This is likely because such small sample does not have the power to rule out a real difference and avoid a type-two error (19). On the other hand, LLR, LAR (in all patients and stratified patients), PAR had a log-rank test with P<0.05. However, the wide CIs limit statistical conclusions. Another method of comparing Kaplan-Meier curves is using the HR, which gives a relative event rate in the groups (19). In this sense, only LAR was an independent prognostic indicator for OS in cHL patients. Nevertheless, it should be noted that given the relatively small number of cHL patients, the multivariable Cox proportional hazard regression model applied in this study may be too conservative or lack enough power to distinguish the prognostic indicators in terms of survival outcomes. More studies in larger patient populations are needed to improve predictions and more accurately estimate effect size, let alone to demonstrate clinical value. Furthermore, the impact of different treatments on patient could modulate the prognostic value of LAR. Thus, studies of patients with various types of treatment or more aggressive treatments are needed to evaluate the predictive capability of LAR in the prognosis of cHL.
There are also strengths in the study. The sample was based on populations from Southern Mexico, adding to the literature on cHL, which is primarily conducted in Europa, Asia, and Northern American people. Furthermore, data from this study was collected from standardized protocols and rigorous quality control. Whether the results obtained in this study could be applied to other regions needed further validation since the optimal cut-off point of LAR might fluctuate as the evaluated population differed. If the prognostic value of LAR is confirmed, a multi-center prospective study to characterize and validate the LAR for predicting worsening in patients with cHL is the next step.
Conclusions
This study suggested that LAR might be a potential biomarker for predicting OS in patients with cHL, a tool with low-cost, non-invasive, and easy to obtain in clinical practice. Due to the fact that this study had a limited number of patients, studies involving more subjects are required.
Acknowledgments
We acknowledge the medical staff at clinical laboratory of UMAE H. E. No. 14.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://aob.amegroups.com/article/view/10.21037/aob-23-24/rc
Data Sharing Statement: Available at https://aob.amegroups.com/article/view/10.21037/aob-23-24/dss
Peer Review File: Available at https://aob.amegroups.com/article/view/10.21037/aob-23-24/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-23-24/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethical and research committee from the UMAE H. E. No. 14, Mexican Institute of Social Security (IMSS) (Registration number: R-2021-3001-103). Informed consent was waived given the retrospective nature of the research.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Brice P, de Kerviler E, Friedberg JW. Classical Hodgkin lymphoma. Lancet 2021;398:1518-27. [Crossref] [PubMed]
- Xie Z, Zhou H, Wang L, et al. The Significance of the preoperative lactate dehydrogenase/albumin Ratio in the Prognosis of Colon Cancer: a retrospective study. PeerJ 2022;10:e13091. [Crossref] [PubMed]
- Aday U, Böyük A, Akkoç H. The prognostic significance of serum lactate dehydrogenase-to-albumin ratio in colorectal cancer. Ann Surg Treat Res 2020;99:161-70. [Crossref] [PubMed]
- Feng JF, Wang L, Yang X, et al. Prognostic value of lactate dehydrogenase to albumin ratio (LAR) in patients with resectable esophageal squamous cell carcinoma. Cancer Manag Res 2019;11:7243-51. [Crossref] [PubMed]
- Gao S, Wu M, Chen Y, et al. Lactic dehydrogenase to albumin ratio in prediction of unresectable pancreatic cancer with intervention chemotherapy. Future Oncol 2018;14:1377-86. [Crossref] [PubMed]
- Şumnu Ş, Mehtap Ö, Mersin S, et al. Serum calprotectin (S100A8/A9) levels as a new potential biomarker of treatment response in Hodgkin lymphoma. Int J Lab Hematol 2021;43:638-44. [Crossref] [PubMed]
- Koyama S, Fujisawa S, Watanabe R, et al. Serum ferritin level is a prognostic marker in patients with peripheral T-cell lymphoma. Int J Lab Hematol 2017;39:112-7. [Crossref] [PubMed]
- He J, Yin H, Xia Y, et al. Prognostic nutritional index, a novel biomarker which predicts worse prognosis in diffuse large B cell lymphoma. Leuk Res 2021;110:106664. [Crossref] [PubMed]
- Zhao Y, Shi Y, Shen H, et al. The prognostic value of platelet-lymphocyte ratio and neutrophil-lymphocyte ratio in the treatment response and survival of patients with peripheral T-cell lymphoma. Leuk Lymphoma 2020;61:623-30. [Crossref] [PubMed]
- Sato MP, Otsuki N, Kimura T, et al. Predictive factors for malignant neoplasms veiled in deep neck infections. Acta Otolaryngol 2022;142:202-5. [Crossref] [PubMed]
- Romano A, Parrinello NL, Vetro C, et al. Prognostic meaning of neutrophil to lymphocyte ratio (NLR) and lymphocyte to monocyte ration (LMR) in newly diagnosed Hodgkin lymphoma patients treated upfront with a PET-2 based strategy. Ann Hematol 2018;97:1009-18. [Crossref] [PubMed]
- Reddy JP, Hernandez M, Gunther JR, et al. Pre-treatment neutrophil/lymphocyte ratio and platelet/lymphocyte ratio are prognostic of progression in early stage classical Hodgkin lymphoma. Br J Haematol 2018;180:545-9. [Crossref] [PubMed]
- Paydas S, Lacin S, Dogan M, et al. Easier and more explanatory indices by integrating leukocyte lymphocyte ratio (LLR) and prognostic nutritional index (PNI) to IPS systems in cases with classical Hodgkin lymphoma. Leuk Res 2021;107:106586. [Crossref] [PubMed]
- Mirili C, Paydas S, Kapukaya TK, et al. Systemic immune-inflammation index predicting survival outcome in patients with classical Hodgkin lymphoma. Biomark Med 2019;13:1565-75. [Crossref] [PubMed]
- Lue KH, Wu YF, Liu SH, et al. Prognostic Value of Pretreatment Radiomic Features of 18F-FDG PET in Patients With Hodgkin Lymphoma. Clin Nucl Med 2019;44:e559-65. [Crossref] [PubMed]
- Simon Z, Barna S, Miltenyi Z, et al. Combined prognostic value of absolute lymphocyte/monocyte ratio in peripheral blood and interim PET/CT results in Hodgkin lymphoma. Int J Hematol 2016;103:63-9. [Crossref] [PubMed]
- Alcoceba M, García-Álvarez M, Chillón MC, et al. Liquid biopsy: a non-invasive approach for Hodgkin lymphoma genotyping. Br J Haematol 2021;195:542-51. [Crossref] [PubMed]
- Rich JT, Neely JG, Paniello RC, et al. A practical guide to understanding Kaplan-Meier curves. Otolaryngol Head Neck Surg 2010;143:331-6. [Crossref] [PubMed]
Cite this article as: Reyes-Pérez EN, Flores-Cuevas LM, Martínez-Mier G, Chávez-Güitrón LE, Martínez-Jiménez MC, Audelo-Guzmán M, Calderón-García J, Reyes-Ruiz JM. Predictive value of the lactate dehydrogenase-to-albumin ratio (LAR) in classical Hodgkin’s lymphoma. Ann Blood 2023;8:32.


