Patient blood management, past, present and future
The early days—pre-1900
Humans have always been intrigued by blood. As far as documented human history goes, blood has been included in various forms of rituals, either traditional or religious. The history of blood transfusion dates back to the time when humans believed that the blood contained something (which was called with various names such as life-giving force, vigor, soul, life, etc.) that could be transferred from one to another. From the Greek fable of Medea and Aeson (1) to the accounts of the Romans who drank it and bathed in it (2), human history has been studded with mentions of blood and the flawed understanding of its functions.
There is a story of Pope Innocent VIII, who was in a coma. In 1492, the physician of the pope attempted to collect blood from young boys. Although there is no evidence of an actual transfusion in this instance, this shows the understanding and practices related to blood in those times (3,4). Although humans did not completely understand the mechanism of the blood flow in the human body, they made attempts to transfer the mysterious powers of the “red fluid” one way or the other.
Four hundred years after the discovery of pulmonary circulation by Ibn al-Nafis (5,6), William Harvey described the whole circulatory system in 1628, which supported Ibn al-Nafis concept of the blood being intravascular and hence gave birth to the further focused attempts to place items intravenously, be it wine (4) or opium or other drugs (7) in dogs.
Experiments with blood transfusions amongst animals lead us to 1665, when Richard Lower performed the first dog-to-dog transfusion and marked the timestamp as a successful blood transfusion, albeit in animals (8). What followed was a passionate attempt to substitute the recipient with humans, with Jean Denis performing a successful lamb-to-human transfusion on June 15, 1667 (9), the patient’s survival, however, is a bigger mystery than the transfusion itself. This was followed by Richard Lower and Edmund King performing a sheep-to-human transfusion on November 23, 1667 (10). Armed with this magic cure, a few more transfusions of lamb blood were attempted in the coming months of 1667 by Denis. However, with the death of the fourth patient, Denis put a decisive end to these experiments. Although interestingly, on further evaluation, the cause of this particular patient’s death was attributed to his scorned wife, who poisoned him with arsenic (11). With the courts stepping in and banning such future excursions, this marked the beginning of a long transfusion moratorium that spanned nearly 150 years.
In the early 19th century, James Blundell revived this lost art of transfusion medicine with his “Experiments on the Transfusion of Blood by the Syringe” (12). He not only demonstrated the use of syringes to perform transfusion but also announced that only human blood should be used for humans. This ultimately led to the first human blood transfusion on September 26, 1818.
Not all transfusions of human blood were successful, and coagulation of the transfused blood was thought to be the culprit in the failures. The use of defibrinated blood did not solve the problem. Even anticoagulated blood with phosphate solutions by Braxton-Hicks in 1869 (13) did not prevent deaths post-transfusions, which were actually being caused due to the mismatch of blood groups that had not yet been discovered.
By the end of the 19th century, the groundwork was being laid for what is now defined as PBM. Surgeons had begun focusing attention on examining a patient’s blood, minimizing hemorrhage, and tolerating anemia, as blood transfusion was not a therapeutic option (14).
Entry into the modern era of transfusion medicine—1900–1960
The first part of the 20th century was not focused on PBM but rather on the development of a safe product that could be stored and administered at a later time and started with a very important discovery (15). In 1900, Karl Landsteiner observed that the sera of some individuals agglutinated the red blood cells (RBCs) of others. This study, published the following year (16) showed for the first time the cellular differences in individuals from the same species (17). With the discovery of the first three blood groups A, B, and C (later group O) by Landsteiner and group AB by his students (18), the foundation was laid for safe transfusion (15). In 1913, Ottenberg showed the importance of compatibility testing to prevent transfusion “accidents” (19). He was the first to recognize the “universal” utility of type O blood donors (20).
Modern transfusion, however, remained impossible, as all transfusions had to be performed directly from the donor’s arm to the patient arm since there were not yet anticoagulants (21). The solution was to be found separately by two physicians. One of the investigators was the Argentinean doctor Luis Agote. On November 9, 1914, in the medical clinic of the Rawson Hospital, Buenos Aires, he transfused 300 mL of blood collected from an employee, and mixed with 3 g of 25% neutral citrate of sodium solution, to a recipient affected with pulmonary tuberculosis. Unfortunately, Dr. Agote did not publish his results in a medical journal. He gave the story to La Prensa, the leading daily newspaper of Buenos Aires. It was not until the following year that Agote’s work appeared in the medical press (21,22). In the United States (US), the surgeon Richard Lewisohn performed two transfusions with sodium-citrated blood and published in a medical journal in January 1915, two months after Agote, for which he took responsibility for the discovery (21).
In 1937, Bernard Fantus started the first hospital blood bank at Cook County Hospital in Chicago (23). Here, he established the idea of a “blood bank” from which previously deposited blood could be withdrawn. At the beginning of World War II, blood donation programs were greatly expanded (24).
During World War II, the rationale of transfusion to save lives from massive hemorrhage remained the rationale for transfusion (25). However, physician practice changed dramatically once blood was readily available (25). Open-heart surgery, orthopedic joint replacements, liver transplantation, burn treatment, and extirpative cancer surgery all now were possible with blood product support (25). It soon became the norm to order at least a type and crossmatch and transfusion for surgery of any kind (25). Dr. Charles Drew spearheaded the “Plasma for Britain” project with our 14 million units collected by the war’s end (15).
The rationale for transfusion rapidly progressed from exsanguinating hemorrhage to correction of measured hemoglobin and hematocrit. The “10/30 rule” of which generations of medical students were taught, stated that patients should not undergo surgery with a hemoglobin value of <10 g/dL or a hematocrit <30% (26). It’s important that changes in practice cannot be overstated. This changed the reason to transfuse from blood loss to a pre-set level of unacceptable anemia (27). By the 1950s, physicians ‘knew’ that blood was safe if it was typed properly (28). Moreover, if they followed the “10/30 rule”, there was no reason not to provide a patient with a transfusion (29). The end result was an era of cavalier, rule-based transfusion with little if any, consideration for the patient (29).
One of the earliest descriptions of PBM is found in the medical bylaws of Providence Hospital in 1953. The Medical Staff adopted the following “Indications for Blood Transfusion”: (I) to replace needed whole blood volume; (II) for oxygen transport: (i) with an anemic patient, otherwise well must have <7 g/dL hemoglobin, (ii) with an anemic patient with complications affecting oxygenation or who is to undergo anesthesia may be transfused if the hemoglobin is <10 g/dL; (III) for exchange transfusion; and (IV) if fresh whole blood is indicated in rare cases of dyscrasias where a labile element is important. Between 1953 and 1960, the application of these indications resulted in a decreasing “use factor” (units transfused annually divided by the number of patients) from 0.237 to 0.110, or by more than 50%. The stated benefits were the following: limiting transfusions to patients with valid medical indications, protecting patients from the hazards of unnecessary transfusions, and easing the burden of donor recruitment and blood collection (30). This was very forward-thinking when the only relevant transfusion-transmitted infection (RTTI) testing was for syphilis.
Bloodless surgery is a non-invasive surgical method developed by an orthopedic surgeon. In the early 1900s, Adolf Lorenz was known as “the bloodless surgeon of Vienna” (31-34). His use of bloodless surgery was due to his severe allergy to carbolic acid routinely used in operating rooms of the time. His condition forced him to become what was then known as a “dry surgeon” (35). Bloodless surgery, however, would not be widely accepted as standard practice for many years.
Response to call from patients refusing blood for religious and safety reasons—1960s–1980s
The Jehovah’s Witness patients (JWP)
The Jehovah’s Witness (JW) religion was founded by Charles Taze Russell in Pittsburgh in 1872 during the Adventist movement based on a literal millennialist interpretation of the Bible (36). In 1931, the organization officially became known as the ‘JWs’ (36). They share some Christian beliefs but hold some unique views as being politically neutral, do not salute flags, enlist in the military, or vote in public elections. They celebrate neither Christmas nor birthdays and must satisfy their ministry’s minimum monthly time requirement (36).
It was not until 1945 that the governing body of the ‘JW’, the Watchtower Society (WTS) introduced the blood transfusion ban, based on the strict literal interpretation of several scriptural passages of the New World Translation of the Bible (36,37). There are more than 7.5 million JWs globally, and the number has been increasing (36). Later, in the 1970s, JWs distributed WTS blood refusal cards. These cards specify that the owner will not accept blood products under any circumstances. Other religious groups have accepted blood transfusion as a medical necessity; to the authors’ knowledge, no other religious group has banned blood transfusion. Hence, refusing potentially life-saving medical interventions such as blood transfusion raised significant ethical and legal issues (37). This has made it essential that treating physicians apply other bloodless medical and surgical interventions to treat those patients.
The effectiveness of hemoglobin substitutes remains elusive, and their use, at least in the US, remains limited to clinical trials or compassionate release. Nevertheless, the question of whether JWP would accept these products was raised long ago with no clear-cut answer. Some of these products contain plasma products such as albumin. Still, each individual JW would decide whether to accept it or not based on the religious conscience of each individual witness (38).
Methods introduced to avoid blood use, such as maintaining iron stores, optimizing hemostasis, minimizing blood loss, and using cell salvage (CS), laid the foundation of the current practice of PBM. Moreover, restrictive transfusion thresholds were promoted by Dr. Aaron Tobian, at Johns Hopkins, by his landmark study that involved 117 JWP who underwent surgery without transfusion. The inferior outcome was only for those with a postoperative hemoglobin level of 6.0 g/dL or lower (39).
Introduction of CS programs
Cardiovascular surgery became one of the most rapidly expanding medical fields in the mid-1950s. Priming the cardiopulmonary bypass (CPB) circuit with heparinized blood often leads to complications, including transfusion reactions. In 1957, Dr. Denton Cooley performed open-heart surgery without the use of a blood transfusion. In the 1960s use of a non-blood priming disposable oxygenator with 5% dextrose in water under normothermia became common practice (40-42).
After the experience of more than 450 patients where surgery had been performed with a non-blood prime CPB, Dr. Cooley operated on seven JWPs without administration of blood before, during, or after the operation (43). It took bravery to push boundaries when a safety net of allogeneic blood was unavailable on standby. He subsequently documented more than 1,200 JWPs had cardiac surgery safely performed with low mortality (44). Dr. Cooley practiced surgical blood preservation techniques following basic postulates of pre-, intra-, and postoperative considerations (43). Although he entered history for performing the first implantation of a total artificial heart, he considered his primary contribution to medicine “reducing the need for blood transfusions in open-heart operations” (45). He is largely recognized as the ‘father of modern bloodless surgery’.
In the 1970s, Dr. Ron Lapin and anesthesiologist Dr. Fred Garcia established the Institute of Bloodless Medicine and Surgery (46). Dr. Ron Lapin, performed surgeries on severely anemic JWP. The group also explored fluorocarbons as a blood substitute (47). Many other groups worldwide followed suit (48).
Several blood conservation techniques have been utilized to minimize allogeneic blood utilization during and after surgery including perioperative anemia management, preoperative autologous blood donation, intraoperative acute normovolemic hemodilution, and perioperative autologous cell salvage (PACS) (49,50). PACS has the benefits of providing “fresh” blood, avoiding the risk of type mismatch, RTTI, and minimizing the risk of transfusion reactions. PACS reduces the risk of alloimmunization to RBCs, platelet, and leukocyte antigens. Another consideration is for patients with rare blood types or those who developed multiple alloantibodies.
Intraoperative blood salvage is performed by aspirating heparin or citrate anticoagulated blood is from the operative field, separating, washing, and concentrating salvaged RBCs for re-infusion (51). Reinfusion may be delayed for up to six hours and occurs through a 40-micron microaggregate or leukocyte depletion filters. CS either during surgery or postoperatively is usually acceptable to JWP but are matters of individual choice (52). There is a Hospital Information Desk that can connect JWP with Liaison Committees at any time to offer advice (53). Contraindications to CS can be defined as absolute or definitive, principally due to red cell lysis that can cause end-organ damage upon reinfusion. Absolute contraindications include salvaged blood mixing with hypotonic solutions, or toxic solutions (sterile water, hydrogen peroxide, alcohol, povidone-iodine). A long list of relative contraindications encompassed CS admixing with contaminants like body fluids, tissue debris, infectious agents, malignancy, pharmacological agents, particularly hemostatic agents (topical thrombin, fibrin glue), bone cement, as nicely discussed by Esper and Waters (54). Caution should be exercised for postpartum hemorrhage, hemoglobinopathies, and cold agglutinin disease (54). Surgical blood conservation preserves precious allogeneic blood resources, especially during national shortages and limited supply.
Complications of perioperative CS include coagulopathy, transfusion-related volume overload, bacterial contamination, and embolism with air, fat, or cellular macroaggregates (55).
Several national and international professional societies that emphasize PBM recommend establishing written policies and procedures for properly collecting, labeling, storing and infusing the CS collected blood (56-58).
Human immunodeficiency virus (HIV) in the blood supply
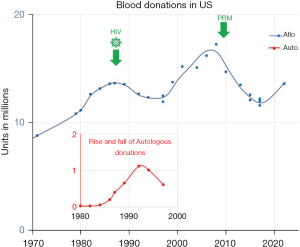
The US national survey of blood collections and transfusions in 1979–1980 found that by converting to voluntary blood donors and implementing a screening test for hepatitis B surface antigen, the national blood supply has become safe (59). There was a doubling in the collection of blood and blood components between 1971 and 1980, thus ensuring an adequate blood supply (60) (Figure 1).
In June 1981, the first reports of a new opportunistic disease in homosexual men emerged (60). By 1983, epidemiological evidence and investigations by the Centers for Disease Control and Prevention strongly suggested that the agent causing acquired immunodeficiency syndrome (AIDS) was transmitted through blood and blood components, or in other words, the agent was a RTTI (63). This was based on two findings: first, AIDS was reported in hemophilia patients who had received antihemophilic factor concentrate transfusion but otherwise did not belong to any previously defined ‘at risk’ group for contracting AIDS; and second, the pattern of AIDS was epidemiologically similar to hepatitis B, another known RTTI (63).
The official recommendations were first promulgated by the Public Health Services, Assistant Secretary for Health for preventing AIDS in March 1983, and the Food and Drug Administration that codified safe practices for blood and plasma collection (63). These included questions to eliminate high-risk groups such as recent immigrants from Haiti, intravenous (IV) drug users, and those with exposure to patients with AIDS or early symptoms of AIDS; but questions about high-risk sexual encounters were largely evaded (60).
A blood test for HIV [known as human T-cell lymphotropic virus (HTLV)-III at that time] became available in 1985, and immediately all donated blood units began to be tested for HIV antibodies (63). The ensuing “look back” to identify patients who might have received HIV—positive blood units and the many subsequent court cases have kept the public fearful for many years of the safety of blood transfusions (59).
The HIV epidemic had wide-ranging effects on the US national blood supply. The public, in general, as well as patients and blood donors in particular, had concerns about the safety of the blood supply and acquiring HIV infection through blood transfusion or blood donation (59). Physicians, increasingly aware of the risks of HIV and other RTTIs, started reevaluating the risks versus benefits of transfusions and turned to substitute therapies that also included avoiding blood transfusions altogether (64).
A series of national surveys were conducted to evaluate the transfusion and blood collection activities in US post-discovery of HIV/AIDS. The most striking finding of surveys were conducted between 1982–1988 (64). was the discovery that the number of allogeneic blood unit collections peaked in 1982 and declined steadily through 1988. Perioperative autologous donation (PAD) by patients scheduled for elective surgery increased rapidly from 1984 through 1987. Linear projections based on the national rates for RBC transfusions between 1971 to 1984 suggested that transfusion rates were 15% lower in 1987 and continued to decline in 1988.
Many of the “basic indications” for transfusions started to be reconsidered. A National Institutes of Health consensus-development conference in 1984 on “Fresh-Frozen Plasma: Indications and Risks” concluded that plasma should not be used as a nutritional source or volume expander (65). Concerns about the blood supply were not confined to the US. In 1997, Justice Krever’s Commission report led to 50 recommendations and results in the implementation of blood conservation initiative in Canada.
These changes were a reflection of the increasing concern on the part of physicians and patients about the risk of acquiring AIDS through blood transfusions. The changes coincided with advances in knowledge about transfusion-associated AIDS. Considerations of blood safety, particularly about the risks of RTTI, were given more weight in the transfusion decision; as a result, allogenic RBC transfusions declined, and increasing numbers of patients deposited their own blood in advance of surgery for autologous blood transfusion (ABT).
Rise and fall of ABT
Transfusion of blood has been an important treatment modality in medical practice. But at times, it has been questioned and re-evaluated in response to numerous concerns about the safety of allogeneic blood (66), increased mismatch between the availability and demand of blood (67), and growing knowledge about the immunomodulatory effects of allogenic transfusions (68).
Initial concerns were of transmission of hepatitis viruses, HIV, and HTLV from receipt of allogenic transfusions. The introduction of screening tests for RTTI of high sensitivity has reduced the risk of transfusion but added to the cost of processing each unit (69). In recent years, several modifications such as leukodepletion, pathogen reduction technology, and irradiation have been introduced to further reduce the risk allogeneic transfusions; however, each adds to the cost of one blood unit (69). It has been calculated that the cost of blood to a patient is three to five times that of hospital acquisition costs when direct and indirect costs are included (70).
ABT re-emerged as the new standard of transfusion practice and was endorsed widely as the safest form of transfusion in the 1980s when it was widely endorsed as the safest form of transfusion. The primary driving force for the re-emergence of ABT was the risk of transfusion-transmitted infections such as HIV and to conserve the precious blood inventory (71).
The possibility that autologous blood donors harbor viral agents, including HIV, was never considered initially. However, as the seroprevalence of HIV continued to increase and ABT continued to grow, the risk of autologous blood donors being infected with HIV or another RTTI, its collection, storage, and transfusion raised complex medical, ethical, and legal issues (72). This included, “Is there medical utility of ABT in HIV-positive patients? Is there an ethical mandate to exclude such ‘dangerous’ autologous units from the blood inventory? Is there a risk of HIV-positive autologous unit to be accidentally transfused to an HIV-negative recipient? Should infectious disease marker testing of autologous blood be mandated? Are universal precautions adequate to protect personnel involved in the handling of HIV-positive units?”. A survey of the American Red Cross Blood Services between April 1993 and March 1994 showed that, on a unit-by-unit basis, the HIV positivity rate for autologous blood donations was 1 in 10,000 as compared to 1 in 25,000 for allogeneic blood donations (72).
Though ABT reduces the risk of alloimmunization and related adverse transfusion reactions, but it does not reduce the patient’s risk of receiving the wrong unit of blood nor guard against bacterial infection of the donated units, a risk similar to allogeneic blood (73). There are both advantages and disadvantages of ABT (Table 1).
Table 1
| Advantages | Disadvantages |
|---|---|
| Reduced risk of RTTI | More expensive |
| Reduced risk of alloimmunisation | Needs additional coordination between surgery team and transfusion team |
| Safe transfusion in patients with multiple alloantibodies or rare blood group | Complex logistics of storage and issue |
| Reduced allogenic blood transfusions | Higher risk for clerical errors |
| Conservation of blood inventory | High rate of discard |
| Acceptable by some JWP | Iatrogenic anemia may lead to transfusion |
| Reduced risk of allergic transfusion reactions | Risk of bacterial contamination |
ABT, autologous blood transfusion; RTTI, relevant transfusion-transmitted infection; JWP, Jehovah’s Witness patients.
Clinical trials of ABT are mostly small and low quality, and hence the evidence of risks outweighing the benefits is low on evidence. Systematic reviews of published trials found no significant reduction in allogenic transfusions compared to standard care or other blood conservation techniques, and safety of acute normovolemic hemodilution (ANH) remains unclear (74). Given the present-day safety of allogenic transfusions from RTTI, the rationale, safety, and cost-effectiveness of routine ABT have been severely questioned, and the British Committee for Standards in Haematology guideline on ABT only recommends its use in “exceptional circumstances” (75).
Rise of blood conservation programs and professional societies 1990s–2010
As discussed earlier, the emergence of AIDS was an era of concern for transfusion. This led to efforts to rationalize the need for a blood transfusion, renewed interest in blood alternatives, and bloodless surgery. With Denton Cooley performing the open-heart surgery without the use of blood transfusion, new horizons began to appear that showed a glimpse of a world devoid of complications of blood transfusion (45). As the 20th century ended and the 21st century began, blood management was now beginning to be considered as a “standard of care” and various organizations embarked on the journey to support research into this area. The notable professional societies that heralded this change toward blood management are listed in Table 2. In 1988, Dr. James Isbister from Australia put forward his point of view about a paradigm shift in blood transfusion (74) where he advocated that “transfusion services should be recipient oriented”. Continuing this journey, in 2005, he coined the term “Patient Blood Management” (PBM) (75) and drove home the point of changing the focus from the product to the patient. Notable contributions in PBM have been made by the Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis (formerly the Network for the Advancement of Transfusion Alternatives) NATA which was established in 1998 (76) and National Blood Authority, Australia (NBA) which was formed by the National Blood Authority Act [2003] (77).
Table 2
| Professional society | QR code |
|---|---|
| International Foundation for Patient Blood Management (IFPBM) |  |
| Society for the Advancement of Blood Management (SABM)—includes SABM Administrative and Clinical Standards for Patient Blood Management Programs 5th Edition |  |
| Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis (NATA) |  |
| AABB and The Joint Commission PBM Certification |  |
PBM, patient blood management; QR, quick response; AABB, Association for the Advancement of Blood and Biotherapies.
The Society for the Advancement of Patient Blood Management (SABM) [established as Society for the Advancement of Bloodless Medicine and Surgery (SABMS) in 2000] has brought in various PBM programs and free access standards that have even been translated into multiple languages, including Portuguese, Korean, Chinese, Nepalese and Spanish. Joining forces in this endeavor, Association for the Advancement of Blood and Biotherapies (AABB) and The Joint Commission have partnered to provide the PBM Certification in order to reduce blood wastage and improve patient outcomes. An emphasis is now made that transfusion should only occur when appropriate as there is increased morbidity and mortality with each unit.
Current era and a look ahead—today and beyond
Data mining
A high-quality transfusion related data collection, including well defined patient outcomes, trough systematic approach of data mining, and big data analysis can support a next phase of PBM evolution. Historical observational data modulated both increase or decrease of transfusion utilization. The mortality rate of wounded men who reached hospitals alive in World War II was less than half compared with the great war (World War I); in part attributed by the availability and liberal use of blood and plasma (78). Development of sustained blood donation programs increased general blood availability. In turn, readily available blood for transfusion supported the evolution of many surgical advances and supported therapy for hematologic diseases and cancer. As transfusion indications broadened and shifted from preventing exsanguination to maintaining the perioperative hemoglobin target. An increased number of transfusions surfaced additional transfusion-associated risks such as RTTI, adverse events, and errors. Change in perceived benefit/risk ratio of transfusion in turn, shifted the decision pendulum toward more conservative allogeneic transfusion and rise in ABT (Figure 1). Improving transfusion safety through RTTI testing in the next wave reassured community of blood safety. This observation led to a rebound of allogeneic transfusions and a steady decrease in hospitalizations reporting ABT (approximately 20 folds decrease over 20 years from 1995 to 2015). Preoperative autologous donations increased from 1984 peaked in 1994, and then steadily declined (49).
Perceived benefits and risks evolved through observations, observational studies and randomized clinical trials (RCTs) (79). Multiple large RCTs support a restrictive hemoglobin threshold for RBC transfusion (80,81). Although RCT less prone to bias, have impediments due to insufficient sample size, inadequate patient selection, and poor endpoint definitions. In addition, overlooking important covariates (that modify exposure and outcome) and confounders (that influence both the exposure and outcome, causing a spurious association) could be detrimental to the meaningful interpretation of results (82-84).
With a standardized electronic multicentric data collection and sharing—the holy grail of transfusion medicine is within reach (85). A controlled data collection obtaining reliable and actionable real-world data allows goal-directed analysis. Hospital clinical information system (CIS) and laboratory information system (LIS) with well-defined data acquisition can collect a massive amount of relevant data. In addition, standardized hospital data can be combined in national or international data repositories that can be analyzed to make more meaningful observations (86-88).
Building a data analytics platform accounting for patient privacy, can develop quality tools to improve patient outcomes and treatment efficiency (89). Big data analysis can emulate a RCT with a retrospective analysis framed around specific rules eliminating bias (90).
A proactive approach to documenting the effects of PBM implementation is supported by defining PBM-related metrics, hospital transfusion practice audits correlated with benchmarking, and patient outcomes (91). Internal and external benchmarking for selected surgical procedures add valuable context to implementing best practices alerts (BPAs) (92). A study analyzing allogeneic transfusions and the clinical outcomes in over 400,000 hospital admission from 2010 to 2018 documented an absolute risk reduction, a drop in the number of blood products transfused, and a decrease in adverse events with PBM implementation (92). However, more granular information could be derived from correlating multivariate clinical outcomes. The expanded list of patient outcomes in big data collections can be obtained through International Classification of Diseases, 10th Revision (ICD-10) codes and includes but is not limited to mortality, morbidity, infections (sepsis, pneumonia), acute renal failure, acute myocardial infarction, acute ischemic stroke, thrombotic outcomes, length of hospital stay/time in the intensive care unit, hemovigilance data, transfusion reactions, transfusion-associated cardiac overload, transfusion-associated acute lung injury, and RTTIs (93,94).
Considerable heterogeneity is associated with transfusion practice globally. This variability of acceptable transfusion practice presents an opportunity for PBM programs to align with best practices and reduce transfusions (88). A next-generation benchmarking tool includes PBM real-time data of individual patient records and individual provider generated data visualization of “Patients Like Mine” visualization in comparison to peer group, adding to learning and better decision making (95-97).
Digital algorithms and artificial intelligence (AI)
Substituting legacy methods, AI has reformed our world. AI has been harnessed to empower the applications of PBM. Efforts have been employed to incorporate machine learning into the clinical decision-making for transfusion, using data sets (98,99) and stochastic dynamic programming to predict need and optimize ordering (100,101) Methods studied include logistic regression, random forests, artificial neural networks (ANNs), and gradient boosting (102). For example, ANNs offer an acceptably reliable mechanism to evaluate the potential for perioperative transfusion across a broad spectrum of surgical interventions preoperatively including reducing unnecessary crossmatching and the number of times when there are out-of-stock blood components (94,103). AI will not replace informed clinical decisions but will support the medical practice, including transfusion medicine to be more cost-effective and personalized.
Some organizations supporting PBM have implemented smart applications as less sophisticated and probably less costly methods for PBM applications. The blood component app, is a mobile and web-based application created by a collaboration between the NHS Blood and Transplant, the National Blood Transfusion Committee, the British Society for Haematology, and the National Institute for Health and Care Excellence. It provides guidelines to support safe and appropriate use of blood components (104). The iTransfuse app is another example of a PBM application developed by the Australian Red Cross (105). Iron deficiency anemia (IDA) algorithm app is another application from Australia provided by the Department of Health & Wellbeing that provides an accessible tool to increase understanding of the diagnosis, investigation, and management of IDA (106). These applications are free, user-friendly, and easily accessible. They can be installed by users on their smart mobile phones. However, consistency in patient care is less likely to be achieved when compared to built-in software (Table 3).
Controversies in PBM—does PBM really decrease transfusion?
Despite the global advocacy for PBM among the World Health Organization (WHO) and the healthcare systems, strong evidence is weak as per the current grading system. No randomized controlled studies evaluated the effect of PBM as a bundle. Nevertheless, are RCTs the only reliable approach to prove the effectiveness of PBM? Is conducting such studies feasible or ethical? In fact, the idea that only randomized, controlled trials produce reliable results is misleading (99), especially since one always needs to consider the unforeseen risks of transfusion.
Specific PBM measures, such as maintaining hemoglobin concentration, optimizing hemostasis, and minimizing blood loss, did not demonstrate enhanced risk-benefit ratio, cost-effectiveness, and improvement of the patient outcome on the RCT level, and much of the research has shown less patient exposure to the blood products without a significant difference in patient outcomes (107-109). Many studies have linked preoperative anemia to poor perioperative patient outcomes (110). It should be noted that trials to reverse anemia with preoperative iron and erythropoietin stimulating agent therapy against liberal transfusion thresholds have not shown a superior patient outcome (111). The Preoperative Intravenous Iron to Treat Anaemia Before Major Abdominal Surgery (PREVENTT) trial compared the effect of IV iron with placebo in anemic patients undergoing abdominal surgery and reported improved hemoglobin levels in the intervention groups. However, both groups had similar blood transfusions and serious adverse events (112). It should be noted that the subgroup analysis revealed a cohort of approximately 30% who were not iron deficient in the first place. A smaller trial of 72 patients in Australia found that IV iron for patients with IDA (ferritin <300 µg/L, transferrin saturation <25%) did reduce perioperative blood transfusion (12% vs. 31%) and shorter hospital length of stay but without any differences in other patient outcomes (113). Adding to that, the cause of anemia cannot be explained in many vulnerable populations. In approximately one-third of elderly patients with anemia, the etiology of anemia cannot be attributed alone to limited hematinic absorption or functional iron deficiency and might not respond to oral or parenteral hematinic replacement. Therefore, it is called “unexplained anemia of the elderly” (61,62).
Most studies compare restrictive vs. liberal thresholds. However, in actual practice, the optimal threshold for hemoglobin might vary among various patient populations and with various medical and surgical interventions. The goal of transfusion is not a target number; it is to provide adequate tissue oxygenation according to the metabolic demands of that specific patient, which is difficult to measure in these studies. Therefore, the use of those markers that show the balance between tissue oxygen delivery and consumption, rather than strict hemoglobin values, recently were suggested as triggers for RBC transfusion (114,115).
The use of CS might maintain hemoglobin levels and reduce allogeneic blood transfusion but could alter hemostatic function, especially when larger volumes are infused, as the processing of salvaged blood causes loss of coagulation factors and platelets as a result of consumption, dilution, activation of fibrinolysis (116). The amount of reinfused salvaged blood volume that could lead to coagulopathy is unknown. A lower number of administered blood products can, however, reduce cost (117). However, calculating the extent of cost-reduction is variable prone to bias (118). Finally, the evidence is much more uncertain about the effect of PBM on plasma and platelet transfusions, and because blood products are of limited resources, wise utilization, regardless of PBM, is required.
Conclusions
While the composition of human blood has not changed since James Blundell performed the first human-to-human transfusions more than two centuries prior, knowledge of indications and appropriate use has evolved through the development of PBM. It encompasses both the judicious use of transfusion as well as the appropriate treatment of those patients for whom blood is not an option. The future is bright for PBM as technological advances allow for high-quality data collection, data mining, and big data analytics that can promote informed PBM healthcare decisions. PBM apps allow global access to guidelines to support safe and appropriate use of blood components to anyone with access to a smartphone. It is encouraging that the most recent National Blood Collection and Utilization Survey data show Shows continued stabilization in transfusions US (119). This marks the first time since 2008 that blood collections in US have not decreased year-to-year. This also suggests that a plateau has been reached for both blood collection and use. PBM programs and restrictive use of transfusions in surgical settings have likely contributed historically to decline in blood use (119). Despite the global advocacy for PBM among the WHO and the healthcare systems, strong evidence is weak as per the current grading system, as no RCTs evaluated the effect of PBM as a bundle (120).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Blood for the series “Patient Blood Management’s Role in Current Healthcare Environment”. The article has undergone external peer review.
Peer Review File: Available at https://aob.amegroups.com/article/view/10.21037/aob-22-45/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-22-45/coif). The series “Patient Blood Management’s Role in Current Healthcare Environment” was commissioned by the editorial office without any funding or sponsorship. R.R.G. served as the unpaid Guest Editor of the series. L.V.V. serves as unpaid member of AABB’s Quality, Regulatory and Management subcommittee, AABB Certified Advanced Biotherapies Professional (CABP) (H), and Blood Banks Association of New York State. N.A. reports honorarium from Terumo BCT and Octapharma, and support from Octapharma for ISBT congress 2023. N.A. serves as unpaid member of ISBT young professional council and AABB cellular therapy standard committee. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bulfinch T. Mythology. New York: Random House; 1855: 111-2.
- Moog FP, Karenberg A. Between horror and hope: gladiator's blood as a cure for epileptics in ancient medicine. J Hist Neurosci 2003;12:137-43. [Crossref] [PubMed]
- Lindeboom GA. The story of a blood transfusion to a Pope. J Hist Med Allied Sci 1954;9:455-9. [Crossref] [PubMed]
- MALUF NS. History of blood transfusion. J Hist Med Allied Sci 1954;9:59-107. [PubMed]
- Haddad SI, Khairallah AA. A forgotten chapter in the history of the circulation of the blood. Ann Surg 1936;104:1-8. [Crossref] [PubMed]
- Al-Ghazal SK, Zubairi R. Ibn Al-Nafis – The First who described the Pulmonary Blood Circulation. Journal of the British Islamic Medical Association. 2022;10:1-6.
- Wren C. An account of the rise and attempts, of a way to conveigh liquors immediately into the mass of blood. Philos Trans R Soc Lond 1665;1:128-30. [Crossref]
- Lower R. The success of the experiment of transfusing the blood of one animal into another. Philos Trans R Soc Lond 1666;1:352.
- Brown H. Jean Denis and transfusion of blood, Paris, 1667-1668. Isis 1948;39:15-29. [Crossref]
- Lower R. An account of the experiment of transfusion, practised upon a man in London, 1667. Philos Trans R Soc Lond 1667;2:557-64.
- Denis J. An extract of a printed letter touching the differences risen about the transfusion of blood. Philos Trans R Soc Lond 1667;2:489-504.
- Blundell J. Experiments on the Transfusion of Blood by the Syringe. Med Chir Trans 1818;9:56-92. [Crossref] [PubMed]
- Braxton-Hicks J. Cases of transfusion, with some remarks on a new method of performing the operation. Guy's Hosp Rep 1869;14:1-14.
- Isbister JP, Pearse BL, Delaforce AS, et al. Patients' Choice, Consent, and Ethics in Patient Blood Management. Anesth Analg 2022;135:489-500. [Crossref] [PubMed]
- Simon T, Gehrie E, McCullough J, et al. editors. Rossi’s Principles of Transfusion Medicine. 6th ed. Hoboken, NJ: John Wiley & Sons Ltd.; 2022.
- Landsteiner K. Über Agglutinationserscheinungen normalen menschlichen Blutes. Wien Klin Wochenschr 1901;14:1132-4.
- Dixon B. Of differential bloods. Science 1984;5:65-7.
- Decastello A, Stuirli A. Ueber die Isoagglutinine im Serum gesunder und kranker Menschen. Munch Med Wochenschr 1902;49:1090-5.
- Ottenberg R, Kaliski DJ. Accidents in transfusion. Their prevention by preliminary blood examination: Based on an experience of one hundred twenty-eight transfusions. JAMA 1913;61:2138-40. [Crossref]
- Ottenberg R. Studies in isoagglutination: I. Transfusion and the question of intravascular agglutination. J Exp Med 1911;13:425-38. [Crossref] [PubMed]
- Lefrère JJ. Transfusion medicine history illustrated: A historic picture: the first transfusion of citrated blood. Transfusion 2011;51:1140-1. [Crossref] [PubMed]
- Agote L. Nuevo procedimiento para la transfusión de sangre. Anales del Instituto Modelo de Clínica. Médica 1915;1:24-31.
- Abbreviated timelines of great moments in transfusion. AABB News. 2022;11.
- Diamond LK. The story of our blood groups. In: Wintrobe MM. editor. Blood, pure and eloquent. New York: McGraw-Hill; 1980:658-717.
- Spence RK, Erhard J. History of patient blood management. Best Pract Res Clin Anaesthesiol 2013;27:11-5. [Crossref] [PubMed]
- Adams R, Lundy JS. Anesthesia in cases of poor surgical risk. Some suggestions for decreasing the risk. Surg Gynecol Obstet 1942;74:1011-9.
- Mason RL, Zintel HA. Preoperative and postoperative treatment. Philadelphia: W.B. Saunders; 1946.
- Tatarelli G. Transfusion of whole blood and its fractions; proposal for a practical solution of the problem aboard ship and on land; general organization of the service; definition of the technic of selection and of necessary material. Ann Med Nav (Roma) 1950;55:341-59. [PubMed]
- Keynes G. Blood transfusion. Bristol: John Wright & Sons; 1949.
- McCoy KL. The Providence Hospital Blood Conservation Program. Transfusion 1962;2:3-6. [Crossref]
- The New York Times October 26, 1902:7.
- The New York Times September 10, 1906:1.
- The New York Times December 25, 1902:3.
- The New York Times November 22, 1926:3.
- Jackson RW, Pollo FE. The legacy of Professor Adolf Lorenz, the "bloodless surgeon of Vienna". Proc (Bayl Univ Med Cent) 2004;17:3-7. [Crossref] [PubMed]
- Petrini C. Ethical and legal aspects of refusal of blood transfusions by Jehovah's Witnesses, with particular reference to Italy. Blood Transfus 2014;12:s395-s401. [PubMed]
- Muramoto O. Bioethical aspects of the recent changes in the policy of refusal of blood by Jehovah's witnesses. BMJ 2001;322:37-9. [Crossref] [PubMed]
- Bailey R, Ariga T. The view of Jehovah's Witnesses on blood substitutes. Artif Cells Blood Substit Immobil Biotechnol 1998;26:571-6. [Crossref] [PubMed]
- Tobian AA, Ness PM, Noveck H, et al. Time course and etiology of death in patients with severe anemia. Transfusion 2009;49:1395-9. [Crossref] [PubMed]
- NEPTUNE WB. BOUGAS JA, PANICO FG. Open-heart surgery without the need for donor-blood priming in the pump oxygenator. N Engl J Med 1960;263:111-5. [Crossref] [PubMed]
- Zuhdi N, McCollough B, Carey J. Double-Helical Reservoir Heart-Lung Machine Designed for hypothermic perfusion; primed with 5% glucose in water; inducing hemodilution. Arch Surg 1961;82:320-5. [Crossref] [PubMed]
- Cooley DA, Beall AC Jr, Grondin P. Open-heart operations with disposable oxygenators, 5 per cent dextrose prime, and normothermia. Surgery 1962;52:713-9. [PubMed]
- Cooley DA, Crawford ES, Howell JF, et al. Open heart surgery in Jehovah's Witnesses. Am J Cardiol 1964;13:779-81. [Crossref] [PubMed]
- Ott DA, Cooley DA. Cardiovascular surgery in Jehovah's Witnesses. Report of 542 operations without blood transfusion. JAMA 1977;238:1256-8. [Crossref] [PubMed]
- Dr. Denton Cooley Pioneering Heart Surgeon Dies at Age 96. New York Times. 2016 Nov 19; Section D, page 6.
- Church G. No mans blood. New York. National Literary Guild; 1983.
- Tremper KK, Friedman AE, Levine EM, et al. The preoperative treatment of severely anemic patients with a perfluorochemical oxygen-transport fluid, Fluosol-DA. N Engl J Med 1982;307:277-83. [Crossref] [PubMed]
- Kay JH. Letter: Need for blood in open-heart surgery. JAMA 1973;226:1230-1. [Crossref] [PubMed]
- Goel R, Petersen MR, Patel EU, et al. Comparative changes of pre-operative autologous transfusions and peri-operative cell salvage in the United States. Transfusion 2020;60:2260-71. [Crossref] [PubMed]
- Sikorski RA, Rizkalla NA, Yang WW, et al. Autologous blood salvage in the era of patient blood management. Vox Sang 2017;112:499-510. [Crossref] [PubMed]
- Frank SM, Sikorski RA, Konig G, et al. Clinical Utility of Autologous Salvaged Blood: a Review. J Gastrointest Surg 2020;24:464-72. [Crossref] [PubMed]
- Waters JH, Potter PS. Cell salvage in the Jehovah's Witness patient. Anesth Analg 2000;90:229-30. [Crossref] [PubMed]
- Klein AA, Bailey CR, Charlton A, et al. Association of Anaesthetists: anaesthesia and peri-operative care for Jehovah's Witnesses and patients who refuse blood. Anaesthesia 2019;74:74-82. [Crossref] [PubMed]
- Esper SA, Waters JH. Intra-operative cell salvage: a fresh look at the indications and contraindications. Blood Transfus 2011;9:139-47. [PubMed]
- Konig G, Waters JH. Washing and filtering of cell-salvaged blood - does it make autotransfusion safer? Transfus Altern Transfus Med 2012;12:78-87. [Crossref] [PubMed]
- Patient Blood Management Guidelines | National Blood Authority [Internet]. [cited 2023 Apr 4]. Available online: https://www.blood.gov.au/pbm-guidelines
- Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management. Anesthesiology 2015;122:241-75. [Crossref] [PubMed]
- Meybohm P, Froessler B, Goodnough LT, et al. "Simplified International Recommendations for the Implementation of Patient Blood Management" (SIR4PBM). Perioper Med (Lond) 2017;6:5. [Crossref] [PubMed]
- Dawson DA. AIDS knowledge and attitudes for January-March 1989. Provisional data from the National Health Interview Survey. Adv Data 1989;1-12. [PubMed]
- Surgenor DM, Schnitzer SS. The nation’s blood resource: a summary report (NIH publication No. 85–2028). Bethesda, MD: National Institutes of Health; 1985.
- Ershler WB. Unexplained Anemia in the Elderly. Clin Geriatr Med 2019;35:295-305. [Crossref] [PubMed]
- Shander A, Knight K, Thurer R, et al. Prevalence and outcomes of anemia in surgery: a systematic review of the literature. Am J Med 2004;116:58S-69S. [Crossref] [PubMed]
- Curran JW, Lawrence DN, Jaffe H, et al. Acquired immunodeficiency syndrome (AIDS) associated with transfusions. N Engl J Med 1984;310:69-75. [Crossref] [PubMed]
- Knowles S. Blood transfusion: challenges and limitations. Transfusion Alternatives in Transfusion Medicine 2007;9:2-9. [Crossref]
- Consensus Conference: Fresh Frozen Plasma. Indications and risks. JAMA 1985;253:551-3. [Crossref] [PubMed]
- Vanderlinde ES, Heal JM, Blumberg N. Autologous transfusion. BMJ 2002;324:772-5. [Crossref] [PubMed]
- Zhou J. A review of the application of autologous blood transfusion. Braz J Med Biol Res 2016;49:e5493. [Crossref] [PubMed]
- Karger R, Weber C, Schmidt J, et al. Characterization of immune system alterations following preoperative autologous blood donation for elective hip replacement surgery. Transfus Med 2007;17:45-53. [Crossref] [PubMed]
- Walunj A, Babb A, Sharpe R. Autologous blood transfusion. Continuing Education in Anaesthesia Critical Care & Pain 2006;6:192-6. [Crossref]
- Shander A, Hofmann A, Ozawa S, et al. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion 2010;50:753-65. [Crossref] [PubMed]
- Provan D. Better blood transfusion. BMJ 1999;318:1435-6. [Crossref] [PubMed]
- Yomtovian R, Kelly C, Bracey AW, et al. Procurement and transfusion of human immunodeficiency virus-positive or untested autologous blood units: issues and concerns: a report prepared by the Autologous Transfusion Committee of the American Association of Blood Banks. Transfusion 1995;35:353-61. [Crossref] [PubMed]
- Brzica SM Jr, Pineda AA, Taswell HF. Autologous blood transfusion. Mayo Clin Proc 1976;51:723-37. [PubMed]
- Isbister JP. The paradigm shift in blood transfusion. Med J Aust 1988;148:306-8. [Crossref] [PubMed]
- Isbister J. Why should health professionals be concerned about blood management and blood conservation? Updates in Blood Conservation and Transfusion Alternatives 2005;2:3-7.
- NATA ONLINE [Internet]. 2023 [cited 2023 Jul 13]. Available online: https://nataonline.com/mission/
- National Blood Authority [Internet]. [cited 2023 Jul 13]. Overview and Role of the NBA. Available online: https://www.blood.gov.au/about-nba
- Kendrick DB. Blood program in World War II. In: Washington, D.C.: Office of the Surgeon General, Dept. of the Army: For sale by the Supt. of Docs., U.S. G.P.O., 1964:922. [cited 2023 Jun 26]. Available online: http://resource.nlm.nih.gov/0014773
- Trentino K, Farmer S, Gross I, et al. Observational studies - should we simply ignore them in assessing transfusion outcomes? BMC Anesthesiol 2016;16:96. [Crossref] [PubMed]
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409-17. [Crossref] [PubMed]
- Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11-21. [Crossref] [PubMed]
- Faraoni D, Schaefer ST. Randomized controlled trials vs. observational studies: why not just live together? BMC Anesthesiol 2016;16:102. [Crossref] [PubMed]
- Trentino KM, Farmer SL, Isbister JP, et al. Restrictive Versus Liberal Transfusion Trials: Are They Asking the Right Question? Anesth Analg 2020;131:1950-5. [Crossref] [PubMed]
- Trentino KM, Farmer SL, Leahy MF, et al. Systematic reviews and meta-analyses comparing mortality in restrictive and liberal haemoglobin thresholds for red cell transfusion: an overview of systematic reviews. BMC Med 2020;18:154. [Crossref] [PubMed]
- Frey BM, Fischer G. Data collection and data sharing - the holy gral of transfusion medicine and modern bioscience. Transfus Med Hemother 2014;41:336-7. [Crossref] [PubMed]
- Mukhtar SA, Leahy MF, Koay K, et al. Effectiveness of a patient blood management data system in monitoring blood use in Western Australia. Anaesth Intensive Care 2013;41:207-15. [Crossref] [PubMed]
- Norgaard A, De Lichtenberg TH, Nielsen J, et al. Monitoring compliance with transfusion guidelines in hospital departments by electronic data capture. Blood Transfus 2014;12:509-19. [PubMed]
- Cohn CS, Welbig J, Bowman R, et al. A data-driven approach to patient blood management. Transfusion 2014;54:316-22. [Crossref] [PubMed]
- Abouelmehdi K, Beni-Hessane A, Khaloufi H. Big healthcare data: preserving security and privacy. J Big Data 2018;5:1. [Crossref]
- Hernán MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am J Epidemiol 2016;183:758-64. [Crossref] [PubMed]
- Meybohm P, Richards T, Isbister J, et al. Patient Blood Management Bundles to Facilitate Implementation. Transfus Med Rev 2017;31:62-71. [Crossref] [PubMed]
- Goodnough LT, Levy JH, Murphy MF. Concepts of blood transfusion in adults. Lancet 2013;381:1845-54. [Crossref] [PubMed]
- Dunbar NM, Szczepiorkowski ZM. Hardwiring patient blood management: harnessing information technology to optimize transfusion practice. Curr Opin Hematol 2014;21:515-20. [Crossref] [PubMed]
- Pendry K. The use of big data in transfusion medicine. Transfus Med 2015;25:129-37. [Crossref] [PubMed]
- Frank SM, Savage WJ, Rothschild JA, et al. Variability in blood and blood component utilization as assessed by an anesthesia information management system. Anesthesiology 2012;117:99-106. [Crossref] [PubMed]
- Markan-Aurora S, Miller R, Gronemeyer AA, et al. Implementation of a Patient Blood Management Program in a Large, Diverse Multi-Hospital System. Journal of Clinical Outcomes Management 2020;27:123-9. [Crossref]
- Lin H, Metcalf RA, Wilburn J, et al. Sanguine: Visual analysis for patient blood management. Inf Vis 2021;20:123-37. [Crossref]
- Walczak S, Velanovich V. Prediction of perioperative transfusions using an artificial neural network. PLoS One 2020;15:e0229450. [Crossref] [PubMed]
- Levi R, Carli F, Arévalo AR, et al. Artificial intelligence-based prediction of transfusion in the intensive care unit in patients with gastrointestinal bleeding. BMJ Health Care Inform 2021;28:e100245. [Crossref] [PubMed]
- van Dijk N, Haijema R, van der Wal J, et al. Blood platelet production: a novel approach for practical optimization. Transfusion 2009;49:411-20. [Crossref] [PubMed]
- Haijema R, van Dijk N, van der Wal J, et al. Blood platelet production with breaks: optimization by SDP and simulation. Int J Prod Econ 2009;121:464-73. [Crossref]
- Mitterecker A, Hofmann A, Trentino KM, et al. Machine learning-based prediction of transfusion. Transfusion 2020;60:1977-86. [Crossref] [PubMed]
- Linwood SLSmit Sibinga CT. Transfusion Medicine: From AB0 to AI Artificial Intelligence 2022;
- Patient Blood Management - Hospitals and Science - NHSBT [Internet]. [cited 2022 Jun 12]. Available online: https://hospital.blood.co.uk/patient-services/patient-blood-management/
- iTransfuse App | Lifeblood [Internet]. [cited 2022 Jun 12]. Available online: https://www.lifeblood.com.au/health-professionals/learn/itransfuse-app
- Iron Deficiency Anaemia App | [Internet]. [cited 2022 Jun 12]. Available online: https://bloodsafelearning.org.au/resource-centre/other-resources/ida-app/
- Bolliger D, Erb JM, Buser A. Controversies in the Clinical Practice of Patient Blood Management. J Cardiothorac Vasc Anesth 2021;35:1933-41. [Crossref] [PubMed]
- Spahn DR, Schoenrath F, Spahn GH, et al. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: a prospective randomised trial. Lancet 2019;393:2201-12. [Crossref] [PubMed]
- Carson JL, Stanworth SJ, Roubinian N, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev 2016;10:CD002042. [Crossref] [PubMed]
- Meybohm P, Westphal S, Ravn HB, et al. Perioperative Anemia Management as Part of PBM in Cardiac Surgery - A Narrative Updated Review. J Cardiothorac Vasc Anesth 2020;34:1060-73. [Crossref] [PubMed]
- Roman MA, Abbasciano RG, Pathak S, et al. Patient blood management interventions do not lead to important clinical benefits or cost-effectiveness for major surgery: a network meta-analysis. Br J Anaesth 2021;126:149-56. [Crossref] [PubMed]
- Richards T, Baikady RR, Clevenger B, et al. Preoperative intravenous iron to treat anaemia before major abdominal surgery (PREVENTT): a randomised, double-blind, controlled trial. Lancet 2020;396:1353-61. [Crossref] [PubMed]
- Froessler B, Palm P, Weber I, et al. The Important Role for Intravenous Iron in Perioperative Patient Blood Management in Major Abdominal Surgery: A Randomized Controlled Trial. Ann Surg 2016;264:41-6. [Crossref] [PubMed]
- Kalteren WS, Verhagen EA, Mintzer JP, et al. Anemia and Red Blood Cell Transfusions, Cerebral Oxygenation, Brain Injury and Development, and Neurodevelopmental Outcome in Preterm Infants: A Systematic Review. Front Pediatr 2021;9:644462. [Crossref] [PubMed]
- Balegar V KK, Low GK, Nanan RK. Regional tissue oxygenation and conventional indicators of red blood cell transfusion in anaemic preterm infants. EClinicalMedicine 2022;46:101365. [Crossref] [PubMed]
- Bolliger D, Tanaka KA. More Is Not Always Better: Effects of Cell Salvage in Cardiac Surgery on Postoperative Fibrinogen Concentrations. J Cardiothorac Vasc Anesth 2020;34:2383-5. [Crossref] [PubMed]
- Xu J, Kinnear N, Johns Putra L. Safety, efficacy and cost of intra-operative cell salvage during open radical prostatectomy. Transl Androl Urol 2021;10:1241-9. [Crossref] [PubMed]
- Bell CM, Urbach DR, Ray JG, et al. Bias in published cost effectiveness studies: systematic review. BMJ 2006;332:699-703. [Crossref] [PubMed]
- Free RJ, Sapiano MRP, Chavez Ortiz JL, et al. Continued stabilization of blood collections and transfusions in the United States: Findings from the 2021 National Blood Collection and Utilization Survey. Transfusion 2023;63:S8-S18. [Crossref] [PubMed]
- Mueller MM, Van Remoortel H, Meybohm P, et al. Patient Blood Management: Recommendations From the 2018 Frankfurt Consensus Conference. JAMA 2019;321:983-97. [Crossref] [PubMed]
Cite this article as: Gammon RR, Almozain N, Jindal A, Nair AR, Vasovic LV, Bocquet C. Patient blood management, past, present and future. Ann Blood 2024;9:7.