
Perioperative anaemia management
Introduction
Anaemia is common finding in patients who are planned for surgical intervention or undergoing emergency surgery and the incidence may be as high as a third of patients pre-surgery (1). Perioperative anaemia is associated with poorer outcomes in surgery even if it is mild in severity (2,3). Anaemia acts synergistically with other comorbidities, i.e., heart and renal failure, in what is known as cardiorenal anaemia syndrome (4) further worsening patients’ outcomes.
Nonetheless, anaemia is not just an ‘innocent bystander’, and if left unmanaged, it can severely affect patient outcomes, quality of life and impose a significant burden on the healthcare system (5). The National Institute for Health and Care Excellence [2015] guidelines (6) therefore state that all non-urgent surgery should be delayed to allow optimization of anaemia. One of the strategies to manage such perioperative anaemia may be blood transfusion and restoration of hemoglobin level. But transfusion has multiple associated complications including alloimmunization, transfusion-transmitted diseases, transfusion reactions, etc. Blood is also a limited and costly resource. So, in the last few decades the focus has shifted from blood transfusion and transfusion threshold concept to the importance of diagnosing, treating and preventing, the underlying causes of anaemia. In the recent concept of health care landscape, patient blood management (PBM) in surgical cases has been aimed at individual patient centered anaemia management to optimize patient outcomes. Adequate treatment of anaemia reduces the need for transfusion along with its associated risks and reduces the length of stay, consequently lessening health-care expenditure (7).
Epidemiology—prevalence of perioperative anaemia
Preoperative patients present with anaemia at a rate higher than the general population. The prevalence of preoperative anaemia varies according to the medical or surgical condition being treated and demographic variables such as age, gender, and geographical region. Observational study results for preoperative anaemia prevalence differ based on study exclusion and inclusion criteria such as exclusion of specific patient types (e.g., obstetric, pediatric, transplant) and inclusion of emergency or minor surgeries. Additionally, the definition of anaemia utilized affects the study’s estimate of prevalence. The majority of studies in the literature use the World Health Organization (WHO) definition of anaemia: hemoglobin less than 130 g/L for men and less than 120 g/L for women. However, some studies use a criterion of hemoglobin less than 130 g/L for both males and females. Since females generally have a smaller body surface area and lower total blood volume compared to males, an equivalent loss of blood volume during surgery represents a higher proportion of hemoglobin mass lost and a concomitantly higher likelihood of red blood cell (RBC) transfusion. Therefore, utilizing a criterion of hemoglobin less than 130 g/L for preoperative anaemia in females may be more appropriate.
Results from the International Surgical Outcomes Study (ISOS) showed 30.1% of adult elective inpatient surgery patients in 27 countries had preoperative anaemia (8). This result is comparable to the preoperative anaemia prevalence of 27.6% found in a large retrospective study of Chinese adult patients (9), 28.7% reported in a large prospective European cohort study of adult non-cardiac, non-obstetric, non-neurological surgery patients (10), and 47.8% seen in a large prospective observational study of adult non-cardiac, non-obstetric surgery patients across South Africa (11). Anaemia prevalence is increased in the elderly, in females, and in less wealthy regions (9,12).
Preoperative anaemia prevalence differs significantly based on type of surgery. Patients undergoing elective total joint arthroplasty for knee or hip showed preoperative anaemia prevalence of 19.6% in a retrospective review of 15,722 patients (13). Preoperative anaemia is much more common in surgery after hip fracture, with prevalence of 65% shown in retrospective review of 34,805 patients in the National Surgical Quality Improvement Program (NSQIP) database (14). The prevalence of preoperative anaemia in patients undergoing colectomy for colon cancer is significant, and was found to be 50.4% in a retrospective review of 35,243 patients in the NSQIP database (15). For the adult cardiac surgery patient population, preoperative anaemia prevalence from 15% to 54% has been reported (16,17), with most studies reporting a prevalence between 25% to 40% (18,19).
Pathophysiology of perioperative anaemia
While preoperative anaemia may result from any of a number of pathologies, the majority fall under acute hemorrhage, chronic blood loss, chronic inflammation, chemotherapy and radiotherapy, and nutritional deficiencies, the most common of which is iron deficiency.
Acute hemorrhage and chronic blood loss
Preoperative anaemia can result from acute hemorrhage in specific emergency surgery settings, e.g., ruptured abdominal aortic aneurysm, traumatic injury. Chronic blood loss may occur with gastrointestinal disorders such as ulcers, polyps, or colorectal cancer; tumors of the kidney or bladder; and heavy menstrual bleeding.
Chronic inflammation
Anaemia of chronic inflammation occurs with systemic inflammation and activation of immune mediators seen in a multitude of disease groups including chronic kidney disease, malignancy, autoimmune diseases, and congestive heart failure, among others (20). Activated immune mediators impede erythropoiesis by inhibiting synthesis of erythropoietin (EPO) by the kidneys and decrease the stimulatory effect of EPO on erythroid progenitor cells (17,21). Upregulation of cytokines induces hepcidin, inhibiting absorption of dietary iron and promoting iron retention in the storage pool (reticuloendothelial cells) (12,20).
Chemotherapy and radiotherapy
Myelosuppressive chemotherapeutic drugs cause anaemia through direct impairment of hematopoiesis in the bone marrow (22). Cytotoxic drugs with nephrotoxic side effects (e.g., platinum-containing agents) can cause anaemia by decreasing synthesis of EPO by the kidneys (22). Radiation therapy damages the bone marrow, reducing production of erythrocytes (23).
Absolute iron deficiency
Absolute iron deficiency describes depletion of total-body iron stores due to (I) inadequate iron intake relative to physiologic requirements, (II) defective absorption, or (III) chronic blood loss. In developing countries, iron deficiency anaemia may result from blood loss associated with certain parasitic infections, such as malaria, schistosomiasis, or hookworm infection (24,25). Reduced absorption may occur due to atrophic gastritis or upregulated levels of hepcidin in chronic inflammatory states (24).
Functional iron deficiency
While the storage pool of iron resides in the liver, spleen and lymph nodes, the functional pool of iron is found within RBCs, bone marrow and cardiac and skeletal muscle (26). Functional iron deficiency results from chronic inflammation and the ensuing cytokine and hepcidin release. Hepcidin not only decreases iron absorption in the duodenum but also causes iron retention within the storage pools through blockage of ferroportin (26). Thus, even in the presence of normal levels of storage iron, functional iron deficiency can lead to anaemia.
Other nutritional deficiencies—vitamin B12 and folic acid
Nutritional deficiencies of vitamin B12 (cobalamin) or folic acid, both essential for erythropoiesis, lead to anaemia. Vitamin B12 deficiency occurs due to poor nutrition or impaired absorption. Intrinsic factor, produced in the stomach and required for absorption of vitamin B12 in the distal ileum of the small intestine, may be deficient due to autoimmune pathology or gastric achlorhydria associated with Helicobacter pylori infection or gastric resection/bypass surgeries (21,27). Nutritional deficiency of folic acid results from inadequate intake or malabsorption due to intestinal diseases, previous surgical resection or bypass of intestine, excessive alcohol consumption, or some medicines (e.g., phenytoin, carbamazepine, gabapentin) (21,28).
Causes of intraoperative and postoperative anaemia
Intraoperative and postoperative anaemia may result from acute normovolemic hemodilution (ANH) techniques for blood conservation or from infusion of priming solutions through the cardiopulmonary bypass circuit prior to cardiac surgery. Blood loss during and after surgery are also major causes of intraoperative and postoperative anaemia.
Outcomes of perioperative anaemia
The association of anaemia with perioperative morbidity and mortality is well described in the literature. Risk for perioperative morbidity and mortality is also correlated with patient American Society of Anaesthesiology (ASA) score, and perioperative anaemia is associated with allogeneic RBC transfusion, an independent risk factor for infection and other morbidities. Allogeneic RBC transfusion and patient preoperative ASA score are confounding factors in most studies of the effect of perioperative anaemia on morbidity and mortality. Some but not all studies have adjusted for these confounding factors in analysis to establish perioperative anaemia as an independent risk factor for morbidity and mortality.
Secondary analysis of the ISOS data from 38,770 patients showed increased risk of in-hospital death within 30 days of surgery for patients with moderate and severe preoperative anaemia compared to patients without anaemia. All grades of preoperative anaemia were associated with an increased risk of experiencing a complication (e.g., infection, cardiovascular complication, or other complication) (8). Analysis of data from NSQIP database for 227,425 patients undergoing non-cardiac surgery showed higher postoperative mortality and higher incidence of one or more major postoperative morbidities in patients with any grade of preoperative anaemia compared to non-anaemic patients (3). Postoperative complications and mortality after cardiac surgery and their association with preoperative and intraoperative anaemia have been widely studied. A multicenter cohort study of cardiac surgery patients at seven academic hospitals in Canada showed patients with preoperative anaemia had an increased incidence of in-hospital outcomes (e.g., composite of death, stroke, or acute kidney injury) after adjusting for confounding variables including intraoperative RBC transfusion (18). A single-center study of 7,957 cardiac surgery patients who did not receive RBC transfusion intraoperatively evaluated the nadir hematocrit during cardiopulmonary bypass and its association with end-organ dysfunction and mortality. A lower intraoperative nadir hematocrit was associated with worse renal function as measured by estimated glomerular filtration rate, more myocardial injury indicated by higher troponin level, longer postoperative ventilator support, longer hospital stays, and higher mortality (29).
Transfusion vs. management of anaemia
The use of allogeneic blood transfusion to manage anaemia and blood loss is a concept that originated several centuries ago and has changed little over the years. Blood collection has historically lagged demand, resulting in a blood supply that is insufficient to meet transfusion needs. Challenges in supply chain may be related to local causes, this includes a high prevalence of infectious disease, the coronavirus disease (COVID) pandemic or sudden epidemics (30-32).
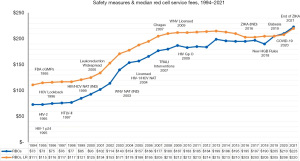
Though the safety of the blood supply has improved markedly with sophisticated testing and public demand for a “zero-risk blood supply” has led to a dramatic decline in the risk of transfusion-transmitted human immunodeficiency virus (HIV), hepatitis C virus, and hepatitis B virus, there is concurrent increase in transfusion related emerging infectious diseases such as West Nile, Zika, and chikungunya viruses (33). Sometimes when newly emergent infections arise, the potential for spread by transfusion is unknown. There is also risk of transfusion related adverse incidents such as transfusion-related acute lung injury (TRALI), transfusion-associated circulatory overload (TACO), hemolytic transfusion reactions, allergic reactions, alloimmunization and immunomodulation, etc. In some instances, these may be life-threatening. The cost of processing of blood units may be another important factor to consider using blood judiciously as additional testing and regulatory measures have contributed to increases (34) (Figure 1). America’s Blood Centers Financial Ratio Survey, 2022 has shown the financial impact of blood transfusion while ensuring its safety.
On the other hand, prevention, while the diagnosis and management if anaemia in perioperative scenario may be time consuming, it has several advantages. In a scenario of a patient with rare blood group and alloimmunization with multiple antibodies against high prevalence antigens or where the patient does not consent for blood transfusion as with the case of Jehovah’s Witness patients, perioperative anaemia management may be the only feasible option.
Preoperative anaemia management
Screening, evaluation, role of pharmacologic agents
A European surgical outcome study of patients undergoing non-cardiac and non-neurological surgery revealed preoperative anaemia to be an independent risk factor for higher in-hospital mortality, longer hospital length of stay, and postoperative intensive care admission (10). Other studies have also found an increased risk of perioperative blood transfusion (35,36). Transfusion, independent of preoperative anaemia, has a dose-dependent increase in complications and mortality of up to 10 folds in patients with lowest predicted risk (37). This underpins the importance of screening targeted elective surgical patients for preoperative anaemia. The screening should be carried out commonly 4 to 8 weeks before the scheduled surgical procedure to allow for sufficient time for investigation, and correction of anaemia (37).
If hemoglobin screening detects anaemia, evaluation should start with a detailed clinical history because anaemia is a symptom of an underlying disease. This evaluation should include nutrition history, history of bleeding especially gastrointestinal hemorrhage. Clinical history is also important to guide the choice of investigation. Of all the causes of anaemia, iron deficiency is the commonest worldwide (38).
Laboratory investigations to evaluate preoperative anaemia should be tailored towards the most common causes based on epidemiological findings. In low-income countries, anaemia due to nutritional deficiencies, and infections/infestations are the most prevalent. Other causes of anaemia include malignancies and chronic renal failure. Therefore, the following laboratory investigations are pertinent for anaemia evaluation: hemoglobin and/or hematocrit, serum ferritin, transferrin saturation, serum vitamin B12, and renal function tests, as well as C-reactive protein (37).
Considering the burden of iron deficiency, any patient irrespective of gender with hemoglobin of less than 130 g/L should be evaluated for iron deficiency. Serum ferritin is the most predictive test for iron deficiency anaemia; and a value of <30 µg/L is suggestive of absolute iron deficiency anaemia with a sensitivity and specificity of 92% and 98%, respectively when compared with an empirical iron therapy or a very invasive bone marrow aspiration biopsy study (39). In the setting of inflammation, a patient with serum ferritin 30 to 100 µg/L, transferrin saturation <20%, and/or C-reactive protein >5 mg/L is considered to have low iron stores (40). Suffice to say that if laboratory evaluation rules out absolute iron deficiency or iron mobilization anaemia (due to inflammation or malignancy), measurement of serum creatinine may indicate chronic kidney disease (41).
In megaloblastic anaemia, the mean corpuscular volume (MCV) is often high, sometimes up to 140 fL. It is often due to deficiency of B12 and/or folic acid. The serum B12 is low in megaloblastic anaemia due to B12 deficiency, but serum folate is not a reliable marker for the investigation of folate deficiency as it is often increased in the presence of vitamin B12 deficiency and due to its lability, it is readily corrected by a nutritious meal (42).
Red cell folate is therefore a more accurate guide to tissue folate status (42). Regarding iron deficiency, a therapeutic trial of oral iron therapy could confirm absolute iron deficiency. However, nonresponse to iron therapy could be because of patient non-compliance (43), ongoing blood losses in excess of oral iron absorption (39), or diminished gastrointestinal absorption of iron due to inflammation (44) or local gastrointestinal condition(s). Therefore, treatment strategies that include iron, B12, folate, or even EPO replacement should be considered.
Nutritional deficiencies must be treated with appropriate supplement(s) before an elective surgical procedure. In the case of megaloblastic anaemia, it may be safer to initiate treatment with both B12 and folic acid. Initial treatment with B12 should be started with injection. Iron-deficiency anaemia should be treated with iron supplementation. Iron deficiency anaemia should be treated preoperatively with oral iron as a first-line therapy if there is enough time to produce good outcome before surgery; and this is usually >6 weeks (40). The readily available oral iron formulations are ferrous gluconate, sulfate, and fumarate in 35, 60, and 100 mg of elemental iron per 300-mg tablet, respectively. Studies have shown favorable patients’ outcomes when oral iron is given preoperatively, but there appear to be no benefit when given postoperatively (45), probably due to decreased absorption following the inflammatory response to surgery (37).
Intravenous (IV) iron therapy is recommended when oral iron is not tolerated due to gastrointestinal side effects or disorders of gastric or intestinal mucosa that interfere with iron absorption. Timing of the surgery where the planned procedure is less than 4–6 weeks could also be an indication for parenteral iron therapy. If iron and vitamins replacement therapy fail, and hemolysis has been ruled out as a cause of anaemia, then the use of erythropoiesis-stimulating agents (ESAs) such as EPO should be considered (46).
ESA therapy can be effective in treating patients with anaemias of inflammation, chronic kidney disease, or patients with iron deficiency anaemia not responsive to iron therapy alone (46). Suffice to say that patients on ESA should also receive iron therapy to provide adequate iron stores for bone marrow red cell production (46).
Autologous blood donation
Autologous blood transfusion in patients is the safest possible blood as it bypasses the complications associated with allogenic transfusion like alloimmunization, transfusion-transmitted diseases and transfusion reactions. Although autologous blood donation was started more than 150 years ago (47), it gained importance in mid-1980s when the risk of HIV transmission increased (48). Another benefit of autologous blood donation is blood conservation and better patient management. Autologous blood transfusion is life-saving in some patients who do not approve allogenic blood transfusion due to religious beliefs (49). Autologous blood can be collected from 5 weeks to 3 days prior before elective surgery and criteria of blood donation is less stringent than allogenic blood donation. However, the risk of donor bacteraemia and contamination of donated blood unit cannot be totally averted via autologous donation (50). Additionally, the risk of mistransfusion, that is autologous blood getting transfused to wrong patient, cannot be eliminated (51).
A 67% wastage rate in autologous blood has been reported in a study by Perez et al. (52). The authors also reported that the preoperative hemoglobin was significantly lower in the autologous donation group compared to control group (133±14 vs. 143±15 g/L, P=0.004). Overtransfusion (i.e., use of the unit because it is available) is a common occurrence in autologous blood units which poses significant threat to patient safety and PBM practices.
Intraoperative anaemia management
Intraoperative cell salvage
It is also known as auto-transfusion, blood salvage or cell saver is widely used in orthopaedic (53-55) and cardiovascular (56,57) surgeries. In this method, the shed surgical blood is collected, washed and reinfused in the patient. Autotransfusion using a blood salvage system can have high levels of free hemoglobin or inflammatory mediators (58,59) and might cause coagulopathy (60).
The blood salvage devices used in various operation theatres usually contains four parts (61): one suction line to collect blood from surgical field; a suction system to deliver the blood to collection reservoir which has anticoagulant [heparin (62) or citrate]; a processing bowl which concentrates the red cells and removes plasma or soluble contaminants to waste bag. Finally, the washed blood is emptied into primary reinfusion bag and moved to secondary bag before transfusion (63).
ANH
The ANH technique utilizes removal a controlled volume of whole blood just prior to surgery along with fluid resuscitation so that hemodiluted blood is lost during operation (64). The collected blood is transfused at the end of operation. It is important to define the safe limit of a lower hematocrit of the patient (65), as that is the critical red cell mass (66). The timing of replacement of autologous blood and maintenance of intravascular volume are important key factors for better patient recovery.
In a meta-analysis (67) it has been concluded that ANH in cardiac surgery has significantly reduced transfusion of allogenic blood (P<0.0001). In a multidisciplinary panel (68), it was discussed that ANH cannot be performed in acute infections and hemodynamically significant arrhythmia. A retrospective study on ANH among cancer patients undergoing major surgeries during the COVID-19 pandemic revealed a favorable outcome without any postoperative adverse events (69). The upper and lower limit of blood that can be removed and lower limit of hemoglobin, however, is yet to be studied.
Anaesthesia and role of the surgeon
Hypotensive anaesthesia (70) can be achieved via epidural blockade or IV anesthesia and one advantage is the decrease of post operative blood loss from spill-over hypotensive effect after surgery. Out of all surgeries; head and neck surgeries like functional endoscopic sinus surgery (FESS), free flap reconstruction, head and neck onco-surgery benefit the most from hypotensive anaesthesia (71,72).
One important factor is maintenance of mean arterial pressure close to 60 mmHg since hypotensive anaesthesia is proven to lower the rate of deep vein thrombosis (DVT) and pulmonary embolism (73). By minimizing intraoperative blood loss, disturbances in coagulability decreases. Circulating anticoagulants like anti-thrombin III level decreases intraoperatively due to hemodilution, and there can be post-operative rebound increase in factor VIII which can cause hypercoagulability due to intraoperative blood loss. In this way, hypotensive anaesthesia by decreasing intraoperative blood loss indirectly helps to prevent DVT. Another reason is, if there is excessive fluid resuscitation, hypothermia can occur which triggers Virchow’s triad predisposing to DVT (74,75).
The use of a tourniquet in total knee arthroplasty is proven to decrease intraoperative blood loss (76). Bipolar sealers denature collagen of the blood vessel wall to decrease intraoperative blood loss (77). Intramedullary femoral plugs (78) made of acrylic cement or bone grafts significantly reduces mean postoperative drainage.
Computer assisted surgeries are proven to result lower hemoglobin loss in many studies when compared to conventional surgery (79). Patient specific instruments by single use cutting jigs are now widely used in orthopedic surgeries and have considerably reduced allogenic blood transfusion rates (80).
Hemostatic agents (Table 1)
Table 1
| Type of hemostatic agent | Mechanism of action | Indication |
|---|---|---|
| Cellulose | Oxidized cellulose is saturated with blood at bleeding site and swells into a gelatinous mass that aids in clot formation | Easy to handle and does not adhere to surgical instruments |
| Collagen | activate the intrinsic pathway of the coagulation cascade and promote platelet aggregation | Widely used at local bleeding site |
| Gelatin | induce hemostasis and activate coagulation cascade through physical properties | To be used in wounds with irregular contour since it will acquire the geometry of the wound and create mechanical tamponade effect |
| Polysaccharide | Its powerful osmotic action dehydrates and gels the blood on contact to accelerate the natural clotting process | Mechanical barrier in local bleeding, not to be used in ophthalmoscopic or neurological or urological surgeries since it expands up to 500 times |
| Thrombin | It Interacts with fibrinogen present in blood at the bleeding site and helps in fibrin clot formation | localized refractory bleeding and during neurological, orthopedic, cardiac, and vascular surgeries |
| Thrombin + gelatin | Stops bleeding by making composite particles of fibrin clot at the bleeding site by biophysical hemostasis | localized refractory bleeding |
| Thrombin + collagen | It causes interaction between human fibrinogen and human thrombin and the physiology of the fibrin clot formation | localized refractory bleeding and vascular surgery |
| Fibrin adhesive | Provide fibrinogen, thrombin and factor XIII at the site of the bleeding that mimics the body’s common pathway of coagulation | Clotting disorder, patients receiving heparin or anticoagulant |
| Albumin + Glutaraldehyde | Glutaraldehyde molecules covalently bond/crosslink the albumin molecules to each other and, on application, to tissue proteins at the repair site, creates a mechanical seal independent of coagulation cascade | Easy to use and most affordable sealant which can be used in mechanical sealing in vascular surgeries |
| Ethylene-glycol polymers | It absorbs large amounts of fluid and expand. And finally close the wound by putting mechanical pressure at the bleeding site | Mechanical sealing of leakage (urology or cardiac surgery) or air leakage in lung operation |
| Cyanoacrylate adhesive | When exposed to anions from skin moisture or wound exudate its monomers quickly polymerize in an exothermic reaction that binds to the most superficial epithelium. It also forms a water-tight barrier stop approximated wound edges creating a cyanoacrylate bridge to allow uninterrupted wound healing | Can be used in coagulopathy as its mechanism of action does not depend on state of coagulation, and has bactericidal property |
The hemostatic agents (82) such as fibrinogen concentrates, vonicog alfa, susoctocog alfa, idarucizumab, andexanet alfa, and argatroban are widely used in peri-operative bleeding which acts via a procoagulant mechanism. In acute trauma surgeries, antidotes to antithrombotic agents have helped to mitigate bleeding emergencies. Topical hemostatic agents help in reducing operating time and bleeding in gynecology patients (83).
In a study conducted on patients with elective orthopaedic surgery who are hemophiliacs with inhibitors, eptacog alfa has been used in initial bolus dose, which helped in overcoming bleeding episodes perioperatively (84). The off-label use of eptacog alfa has also increased in paediatric cardiac surgery as it acts as effective rescue therapy (85). The hemostatic registry has complied the data regarding off-label use of hemostatic agents in cardiac surgery, traumatic surgery, obstetric surgery and medical bleed over 10 years (86). In UK, tranexamic acid is widely used in cardiac, orthopaedic, liver, gynaecological, neurosurgeries, whereas ɛ-aminocaproic acid is widely used in USA. Both tranexamic acid and ɛ-aminocaproic acid are synthetic lysine analogues that act by blocking plasminogen and prevents activation to plasmin, thereby stops fibrin degradation (87).
Post-operative anaemia management
During the post-operative period anaemia management continues to be of importance. Anaemia may lead to cardiovascular and cardiopulmonary complications. Rehabilitation, both in hospital and as an outpatient may adversely affect the speed of recovery if anaemia is present (88). Also, avoiding anaemia will decrease the necessity for red cell transfusions. RBC transfusions are shown to increase length of stay in the hospital and intensive care units, increase risk for infections and increase the rate of mortality (89). Correction even if only partial, of the preoperative hemoglobin level (at least to >100 gm/L) will decrease the chance of significant post-operative blood loss (90). Preoperative halting of anti-platelet agents, non-steroidal anti-inflammatory drugs and anticoagulants also help in decreasing the amount of post-operative blood loss (91). Many of the procedure used preoperatively and intraoperatively may also be used in the postoperative period to prevent or mitigate anaemia.
Sampling
Blood draws for laboratory and point of care testing may lead to iatrogenic anaemia. These tests performed to monitor and manage patients’ care may lead up to 40 mL/day or more of blood loss (92). To curtail this issue protocols may be instituted. The number of draws per day should be limited especially for routine monitoring. If the patient is stable evaluation of the necessity for the tests should be performed. This evaluation may be done every 3–5 days to stop unnecessary more frequent testing. Combining lab tests into one blood tube should also be done as well as sharing of the tubes, if possible, with various sections of the laboratory. Drawing laboratory samples from central lines will also decrease blood wastage. Using smaller sample testing tubes will also save blood by decreasing the volumes of blood sampled for testing by almost 2-fold (93,94).
Nutrition
Post-operative nutrition is important for wound healing and erythrocyte production. IV iron if not given preoperatively can be given post-operatively. The IV iron repletes iron stores quickly. Over the following 2 weeks hemoglobin levels may rise 20 g/L in the anaemic patients (90,95). Vitamin B12 and folate also aid erythropoiesis and maintain red cell mass. Protein deficiency anaemia (aka hypo-proliferative anaemia) may lead to mild to moderate anaemia. This may be seen in multiple chronic diseases such as liver or kidney failure. Post-surgery there may also be malnutrition due to inadequate protein intake. Correcting this deficit may lead to more efficient hemoglobin production and correction of anaemia (96).
Bleeding management
Risk of bleeding increases for up to 48 hours after major surgeries (97). When cardio-bypass pumps are used platelets degranulate while passing through the pumps and become non-functional. During this time if active bleeding occurs platelet transfusions may be required to control the bleeding. Coagulation function assays including prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen and thromboelastography (TEG) may be used to evaluate for the presence of coagulopathies following surgeries. Depending on the deficiency present, specific type of plasma like cryoprecipitated antihemophilic factor (cryo-AHF) cryo-poor plasma, plasma frozen within 24 hours (PF24) or platelets may be given to correct the coagulopathy. If bleeding is present and no coagulopathy is found a surgical exploration may be needed to find a potential anatomic defect (98).
As is performed intraoperatively, post-operative blood salvage is another way to treat anaemia. If the blood volume losses are large the blood may be collected in cell washers, cleaned and reinfused. Reinfusion needed to be done within four hours if blood is processed, collected and stored at room temperature (99). If large volumes especially over 5 units (i.e., 1,000 mL) are given 1:1 transfusions with RBC: plasma should also be instituted to decrease the incidence of transfusion induced coagulopathies (100). For orthopedic surgery patients, techniques can be used to decrease blood loss post-operatively, such as limiting the time that drains are active, using low pressure suctions on drains, and using compression on wound sites or elevating the limb. Each manipulation on its own has limited effect but together may significantly decrease blood loss (101).
Role of pharmacologic agents
As stated previously IV iron may be used to help correct iron deficiency anaemia. Acute blood loss and the inflammatory state following surgery impairs iron absorption and metabolism. IV iron has been shown not to increase the risk of infections, bleeding or other complications (thromboembolisms) as initially hypothesized. Recombinant human erythropoietin (rHEpo) in combination with IV iron can speed up the production of red cells and more quickly correct anaemias. rHEpo is most beneficial for patients who are difficult to crossmatch (i.e., multiple alloantibodies) or with those for whom blood transfusion is not an option (94,102).
If renal insufficiency is present and bleeding is active, treatment can be given to stop the bleeding. If the hemoglobin level is below 70 g/L, RBC transfusion can be given. When hemoglobin levels are between 70–100 g/L blood flows in a laminar fashion and aids interaction of platelets with the endothelial lining. Below this level platelets migrate more to the central area of vessels and are less like to be activated with endothelial damage present. Also, with renal failure platelet function is also impaired, if the platelet count is over 50,000/µL additional platelets transfused may not aid in stopping the bleeding. Desmopressin (DDAVP), in small doses 0.3 µL/kg, causes the release of von Willebrand factor (vWF) from endothelial cells. Dosing with DDAVP can be done only two times in 48 hours secondary to tachyphylaxis. The released vWF aids in platelet function and activation. If bleeding continues after two doses are given, cryoprecipitate may be given to supply additional vWF every 8 hours. Dialysis may also be performed to decrease uremia and improve platelet function. If renal failure is chronic and bleeding continues estrogen can be given and has been shown to increase levels of coagulation proteins (103-105).
For orthopedic surgery patients, techniques can be used to decrease blood loss post-operatively, such as limiting the time that drains are active, using low pressure suctions on drains, and using compression on wound sites or elevating the limb. Each manipulation on its own has limited effect but together may significantly decrease blood loss.
Perioperative anaemia in special group of populations
Particular patient populations, such as pediatric, geriatric, oncologic surgical patients, as well as those for whom transfusion of blood is not an option (such as Jehovah’s Witnesses), present special challenges for the management of perioperative anaemia. For these special cases, there remain a number of vital factors that need to be kept in mind.
First, anaemia is prevalent in paediatric populations and is linked to higher perioperative mortality. Preoperative anaemia occurs in around 46.2% of paediatric patients with accessible haemoglobin measurements with increased prevalence in lower-middle-income countries (106,107). Neonatal postoperative in-hospital mortality is independently correlated with preoperative anaemia (where anaemia was defined as hematocrit level 40%) (108). Although erythropoiesis-stimulating drugs and/or the usage of iron have been associated with decreased transfusion needs in a few paediatric populations, there is not enough information to advocate their routine use in this population (40).
Anaemia is also more common in elderly surgical patients. Increased postoperative morbidity and death are linked to anaemia in this population. Anaemic patients routinely undergo surgery without taking this risk factor for a poor result into account during assessment and care. Allogenic blood transfusions, which are currently the backbone of treatment for anaemia in elderly surgical patients, can have detrimental effects and are increasingly avoided (109).
Anaemia affects one-third of oncology patients at the time of diagnosis and two-thirds of them at the 6-month period. Blood loss, nutritional inadequacies, persistent inflammation, involvement of the bone marrow, hepcidin-driven iron sequestration into macrophages with subsequent iron-restricted erythropoiesis and chemotherapy are some of the causes (110). Regardless of the underlying cause, anaemia continues to be a risk factor for poor prognosis in cancer patients, especially in the perioperative setting, and raises the likelihood of receiving allogeneic blood transfusions, which in turn increases the risk of tumour recurrence and poor patient outcomes. A higher risk of unfavourable outcomes has been independently linked to blood transfusions in cancer patients undergoing surgery (111). Due to reported but contentious potential side effects include tumour progression, tumour recurrence, myocardial infarction, stroke, and early mortality, use of ESAs in cancer patients is strongly contested. Notably, many of these worries stem from studies using high dosages of ESAs for lengthy periods of time in nonsurgical oncologic patients, particularly when aiming for normal or nearly-normal haemoglobin levels. In cancer patients with anaemia, short-term, on-label preoperative erythropoiesis-stimulating drugs treatment is safe according to the most recent research (112,113).
Patients for whom blood transfusion is not an option represent a third patient population that necessitates special treatment. Patients who would refuse blood transfusions out of moral or religious convictions most often members of the Jehovah’s Witness faith as well as those for whom appropriately cross-matched blood is not available fall under this category (114). Epoetin alfa may be dosed daily (300 units/kg) for urgent treatments, however, weekly ESA drugs and IV iron are typically used to address anaemia. However, studies have shown increased morbidity and mortality in patients with severe anaemia who cannot be transfused, and this population may warrant more intensive treatment of anaemia before preforming procedures with the possibility of higher blood loss. In general, outcomes are favourable in these populations when supported with blood conservation techniques. It should be noted that treating anaemia is only a small component of the wider blood conservation procedure that should be employed to effectively and properly care for patients for whom blood is not an option (115,116).
Future directions of perioperative anaemia management
The future of perioperative anaemia management looks bright. An increase in the global awareness of PBM, underpinned by a strong and growing evidence base showing better patient outcomes, the obvious economic benefits and the ethical imperative to manage patients in a way that secures the best possible outcomes while limiting their risk exposure as much as possible, is contributing to PBM becoming an essential element of good patient care overall (117). In addition, the recognition that blood transfusion is one of the five most overused medical procedures and subsequent recommendations to limit blood transfusion to situations where it is truly needed, has bolstered efforts to ensure that it is used more appropriately (118-120). More recently, the WHO has released a policy brief as a call to action for the development of guidelines for global implementation of PBM (121). It is hoped that this will provide the needed impetus for governments, health ministries and private organizations to implement the necessary structures to make this a reality. Many countries around the world, from those of lower to higher income, have already initiated national PBM programs and are increasingly reporting similar benefits to those seen in the early studies (121-123).
A key element of optimisation of perioperative anaemia is the establishment of preoperative anaemia clinics. Depending on locally available knowledge and experience of PBM and anaemia management, this can be run by a clinical nurse practitioner, a general medical practitioner or a specialist (e.g., surgeon, anaesthesiologist, haematologist, etc.). Hospitals should be encouraged to make the investment as the likely future savings, coupled with the ethical imperative of improving patient outcomes, should be more than enough compensation for any costs incurred (124-126).
With increased awareness, a need for increased education has been identified, and it is foreseen that there will be an increase in both formal and informal educational programs (127,128). The ease of use, and increasing adoption of online learning modalities, availability of online resources, as well as the use of virtual platforms for hosting conferences or ad hoc meetings, have brought access to the best education and world class experts on these topics, to the doorstep of almost anyone in the world, that has a smart phone and access to the internet and in a very cost-effective manner (129). It is foreseen that this will expand going forward resulting in high-quality, affordable, credit-bearing educational programs that can be used to satisfy licensure requirement and provide certification in elements of PBM that are required. Further, global, but also country-level guidelines for perioperative anaemia management are still required in many countries. An opportunity exists for skills development in guideline development in many countries as well.
On the diagnostic front, a need has been identified to revisit normal reference ranges and diagnostic cut-off levels for laboratory parameters of anaemia (130,131). Data showing an increase in peri-operative morbidity in women with a hemoglobin <130 g/L, which is higher than the currently accepted “normal” cut-off of 120 g/L, needs to be further elucidated and confirmed. Noting that patients with perioperative anaemia often have iron restriction with normal or increased serum ferritin levels makes the diagnosis of iron deficiency that requires treatment in this group difficult. A lot of uncertainty remains on the precise diagnostic criteria for iron deficiency in conditions where underlying inflammation, malignancy or organ compromise, might lead to laboratory results that might mask iron deficiency. Newer laboratory tests (e.g., reticulocyte hemoglobin content, percentage hypochromic red cells, soluble transferrin receptors, serum and urine hepcidin levels, etc.) are being validated in different surgical and non-surgical settings, and are expected to assist with diagnosing these patients more accurately (130).
The development of new technologies, in terms of cell salvage, easy-to-use and interpret point-of-care testing (e.g., iron studies, coagulation, hemoglobin, etc.), biodegradable sealants and robotic and other forms of surgery assist in limiting blood loss. More accurate ways of measuring patients’ physiological status and their ability to tolerate anaemia by the bedside or through wearable technology to allow for early detection of changes in a patient’s physiological responses that might indicate a need for blood transfusion, will hopefully one day become a reality (130). One can also foresee that assistance by artificial intelligence systems, driven by machine learning algorithms will become more and more accurate in predicting the need for and response to blood transfusion. Finding ways to integrate the data from all these data streams into day-to-day practice such as mobile applications and other digital tools will become important for the clinician and it is likely that support systems that take responsibility for patient monitoring, even if out of hospital, can be expected to become more widespread (132).
Drugs that safely target hepcidin, RBC production and inflammatory cytokines that influence blood production, will hopefully become part of our armamentarium, to address restricted erythropoiesis, as is usually seen in perioperative anaemia, without increasing infectious and other risks.
Conclusions
- Perioperative anaemia is independently associated with poorer outcomes in surgery, which warrants a proactive approach to evaluation and management of anaemia.
- The appropriate approach to treatment of preoperative anaemia will depend on investigation of the patient’s underlying pathophysiology. The most common cause of preoperative anaemia is iron deficiency.
- Appropriate use of iron supplementation combined with ESAs prior to surgery, techniques of intraoperative blood conservation like intraoperative cell salvage and ANH, along with hemostatic agents during intraoperative phase and limiting inappropriate phlebotomy, optimizing nutrition, and judicious utilization of pharmacologic agents during the post operative phase will likely lead to increased adoption of best practices for management of perioperative anaemia.
Acknowledgments
We would like to thank Richard R. Gammon, Medical Director, One Blood, Orlando, FL, USA and Christopher Bocquet, Senior Director, Standards Development and Quality Initiatives, Association for the Advancement of Blood and Biotherapies (AABB) for their substantial contribution and guidance.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Richard Gammon) for the series “Patient Blood Management’s Role in Current Healthcare Environment” published in Annals of Blood. The article has undergone external peer review.
Peer Review File: Available at https://aob.amegroups.com/article/view/10.21037/aob-22-42/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-22-42/coif). The series “Patient Blood Management’s Role in Current Healthcare Environment” was commissioned by the editorial office without any funding or sponsorship. VJL reports payments from Pharmacosmos which made to his institution, and reports honoraria for speaker fees from Acino, Vifor, Pharmacosmos, Austell, and Aspen. VJL also received support for travel and accommodation to meetings and conferences from Acino, Vifor, Pharmacosmos, Aspen, and NATA. VJL serves as an unpaid board member of NATA. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Muñoz M, Laso-Morales MJ, Gómez-Ramírez S, et al. Pre-operative haemoglobin levels and iron status in a large multicentre cohort of patients undergoing major elective surgery. Anaesthesia 2017;72:826-34. [Crossref] [PubMed]
- Clevenger B, Mallett SV, Klein AA, et al. Patient blood management to reduce surgical risk. Br J Surg 2015;102:1325-37; discussion 1324. [Crossref] [PubMed]
- Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet 2011;378:1396-407. [Crossref] [PubMed]
- Lu KJ, Kearney LG, Hare DL, et al. Cardiorenal anemia syndrome as a prognosticator for death in heart failure. Am J Cardiol 2013;111:1187-91. [Crossref] [PubMed]
- Nissenson AR, Goodnough LT, Dubois RW. Anemia: not just an innocent bystander? Arch Intern Med. 2003;163:1400-4. Erratum in: Arch Intern Med 2003;163:1820. [Crossref] [PubMed]
- National Institute for Health and Care Excellence. Blood transfusion 2015;NG24:
- Leahy MF, Hofmann A, Towler S, et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion 2017;57:1347-58. [Crossref] [PubMed]
- Fowler AJ, Ahmad T, Abbott TEF, et al. Association of preoperative anaemia with postoperative morbidity and mortality: an observational cohort study in low-, middle-, and high-income countries. Br J Anaesth 2018;121:1227-35. [Crossref] [PubMed]
- Lin J, Wang C, Liu J, et al. Prevalence and intervention of preoperative anemia in Chinese adults: A retrospective cross-sectional study based on national preoperative anemia database. EClinicalMedicine 2021;36:100894. [Crossref] [PubMed]
- Baron DM, Hochrieser H, Posch M, et al. Preoperative anemia is associated with poor clinical outcome in non-cardiac surgery patients. British Journal of Anaesthesia 2014;113:416-23. [Crossref] [PubMed]
- Marsicano D, Hauser N, Roodt F, et al. Preoperative anaemia and clinical outcomes in the South African Surgical Outcomes Study. S Afr Med J 2018;108:839-46. [Crossref] [PubMed]
- Gómez-Ramirez S, Jericó C, Muñoz M. Perioperative anemia: Prevalence, consequences and pathophysiology. Transfus Apher Sci 2019;58:369-74. [Crossref] [PubMed]
- Greenky M, Gandhi K, Pulido L, et al. Preoperative anemia in total joint arthroplasty: is it associated with periprosthetic joint infection? Clin Orthop Relat Res 2012;470:2695-701. [Crossref] [PubMed]
- Ryan G, Nowak L, Melo L, et al. Anemia at Presentation Predicts Acute Mortality and Need for Readmission Following Geriatric Hip Fracture. JB JS Open Access 2020;5:e20.00048.
- El Ghouayel M, Hamidi M, Mazis C, et al. Surgical Outcomes in Patients With Preoperative Anemia Undergoing Colectomy for Colon Cancer. J Surg Res 2022;273:218-25. [Crossref] [PubMed]
- Hung M, Besser M, Sharples LD, et al. The prevalence and association with transfusion, intensive care unit stay and mortality of pre-operative anaemia in a cohort of cardiac surgery patients. Anaesthesia 2011;66:812-8. [Crossref] [PubMed]
- Nguyen Q, Meng E, Berube J, et al. Preoperative anemia and transfusion in cardiac surgery: a single-centre retrospective study. J Cardiothorac Surg 2021;16:109. [Crossref] [PubMed]
- Karkouti K, Wijeysundera DN, Beattie WS, et al. Risk Associated With Preoperative Anemia in cardiac Surgery. Circulation 2008;117:478-84. [Crossref] [PubMed]
- Kulier A, Levin J, Moser R, et al. Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation 2007;116:471-9. [Crossref] [PubMed]
- Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood 2019;133:40-50. [Crossref] [PubMed]
- Gasmi A, Bjørklund G, Mujawdiya PK, et al. Micronutrients deficiences in patients after bariatric surgery. Eur J Nutr 2022;61:55-67. [Crossref] [PubMed]
- Rodgers GM 3rd, Becker PS, Blinder M, et al. Cancer- and chemotherapy-induced anemia. J Natl Compr Canc Netw 2012;10:628-53. [Crossref] [PubMed]
- Younis M, Iqbal M, Shoukat N, et al. Effect of chemotherapy and radiotherapy on red blood cells and haemoglobin in cancer patients. Science Letters 2014;2:15-8.
- Camaschella C. Iron deficiency. Blood 2019;133:30-9. [Crossref] [PubMed]
- Beyable AA, Berhe YW, Nigatu YA, et al. Prevalence and factors associated with preoperative anemia among adult patients scheduled for major elective surgery at University hospital in Northwest Ethiopia; a cross-sectional study. Heliyon 2022;8:e08921. [Crossref] [PubMed]
- Al-Naseem A, Sallam A, Choudhury S, et al. Iron deficiency without anaemia: a diagnosis that matters. Clin Med (Lond) 2021;21:107-13. [Crossref] [PubMed]
- Rodriguez-Castro KI, Franceschi M, Noto A, et al. Clinical manifestations of chronic atrophic gastritis. Acta Biomed 2018;89:88-92. [PubMed]
- Vidmar M, Grželj J, Mlinarič-Raščan I, et al. Medicines associated with folate-homocysteine-methionine pathway disruption. Arch Toxicol 2019;93:227-51. [Crossref] [PubMed]
- Loor G, Li L, Sabik JF 3rd, et al. Nadir hematocrit during cardiopulmonary bypass: end-organ dysfunction and mortality. J Thorac Cardiovasc Surg 2012;144:654-662.e4. [Crossref] [PubMed]
- Ngo A, Masel D, Cahill C, et al. Blood Banking and Transfusion Medicine Challenges During the COVID-19 Pandemic. Clin Lab Med 2020;40:587-601. [Crossref] [PubMed]
- Williamson LM, Devine DV. Challenges in the management of the blood supply. Lancet 2013;381:1866-75. [Crossref] [PubMed]
- Drackley A, Newbold KB, Paez A, et al. Forecasting Ontario's blood supply and demand. Transfusion 2012;52:366-74. [Crossref] [PubMed]
- Yonemura Y. Emerging infectious disease and transfusion-transmitted infection. Rinsho Ketsueki 2021;62:1296-301. [PubMed]
- United States Department of Health and Human Services. Adequacy of the national blood supply: Report to Congress 2020. Available online: https://www.hhs.gov/sites/default/files/hhs-adequacy-national-blood-supply-report-congress-2020.pdf (Accessed October 10, 2022).
- Freedman J. The ONTraC Ontario program in blood conservation. Transfus Apher Sci 2014;50:32-6. [Crossref] [PubMed]
- Klein AA, Collier TJ, Brar MS, et al. The incidence and importance of anaemia in patients undergoing cardiac surgery in the UK - the first Association of Cardiothoracic Anaesthetists national audit. Anaesthesia 2016;71:627-35. [Crossref] [PubMed]
- Lin Y. Preoperative anemia-screening clinics. Hematology Am Soc Hematol Educ Program 2019;2019:570-6. [Crossref] [PubMed]
- Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014;123:615-24. [Crossref] [PubMed]
- Mast AE, Blinder MA, Gronowski AM, et al. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem 1998;44:45-51. [Crossref] [PubMed]
- Muñoz M, Acheson AG, Auerbach M, et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2017;72:233-47. [Crossref] [PubMed]
- Goodnough LT, Maniatis A, Earnshaw P, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth 2011;106:13-22. [Crossref] [PubMed]
- Hoffbrand AV, Steensma D. Megaloblastic anaemias and other macrocytic anaemias. In: Hoffbrand V, Steensma DP. Hoffbrand’s Essential Haematology. 8th edition. John Wiley and Sons Press, 2020:60.
- Mercuriali F, Zanella A, Barosi G, et al. Use of erythropoietin to increase the volume of autologous blood donated by orthopedic patients. Transfusion 1993;33:55-60. [Crossref] [PubMed]
- Ganz T. Anemia of chronic disease. In: Provan D, Gribben J. editors. Molecular Hematology. 4th edition. John Wiley and Sons Press, 2019:155-60.
- Parker MJ. Iron supplementation for anemia after hip fracture surgery: a randomized trial of 300 patients. J Bone Joint Surg Am 2010;92:265-9. [Crossref] [PubMed]
- Warner MA, Shore-Lesserson L, Shander A, et al. Perioperative Anemia: Prevention, Diagnosis, and Management Throughout the Spectrum of Perioperative Care. Anesth Analg 2020;130:1364-80. [Crossref] [PubMed]
- Yomtovian R, Ceynar J, Kepner JL, et al. Predeposit autologous blood transfusion: an analysis of donor attitudes and attributes. QRB Qual Rev Bull 1987;13:45-50. [Crossref] [PubMed]
- Busch MP, Young MJ, Samson SM, et al. Risk of human immunodeficiency virus (HIV) transmission by blood transfusions before the implementation of HIV-1 antibody screening. The Transfusion Safety Study Group. Transfusion 1991;31:4-11. [Crossref] [PubMed]
- Berg L, Dave A, Fernandez N, et al. Women who decline blood during labour: Review of findings and lessons learnt from 52 years of Confidential Enquiries into maternal mortality in the United Kingdom (1962-2019). Eur J Obstet Gynecol Reprod Biol 2022;271:20-6. [Crossref] [PubMed]
- Goodnough LT, Monk TG. Blood conservation in patients undergoing non-cardiac surgery. Curr Opin Anaesthesiol 2000;13:365-70. [Crossref] [PubMed]
- Shulman IA, Osby M. Storage and transfusion of infected autologous blood or components: a survey of North American laboratories. Arch Pathol Lab Med 2005;129:981-3. [Crossref] [PubMed]
- Perez A, Bakhtary S, Nedelcu E, et al. Overtransfusion of Autologous Blood Identifies Opportunities for Improving Patient Blood Management. Cureus 2019;11:e4202. [Crossref] [PubMed]
- Zou H, Li Z, Sheng H, et al. Intraoperative blood loss, postoperative drainage, and recovery in patients undergoing lumbar spinal surgery. BMC Surg 2015;15:76. [Crossref] [PubMed]
- Dao P, Massin P. Blood management in enhanced recovery after hip and knee replacement. Orthop Traumatol Surg Res 2020;106:S1-5. [Crossref] [PubMed]
- Yu QB. Effect of total knee arthroplasty under computer navigation on intraoperative blood loss and joint function recovery. Zhongguo Gu Shang 2020;33:15-20. [PubMed]
- Wang G, Bainbridge D, Martin J, et al. The efficacy of an intraoperative cell saver during cardiac surgery: a meta-analysis of randomized trials. Anesth Analg 2009;109:320-30. [Crossref] [PubMed]
- Boulos L, Kuebler JD, Angona R, et al. Cell Saver Blood Reinfusion Up to 24 Hours Post Collection in Pediatric Cardiac Surgical Patients Does Not Increase Incidence of Hospital-Acquired Infections or Mortality. J Extra Corpor Technol 2021;53:161-9. [Crossref] [PubMed]
- Vermeijden WJ, Hagenaars A, van Oeveren W, et al. Do repeated runs of a cell saver device increase the pro-inflammatory properties of washed blood? Eur J Cardiothorac Surg 2008;34:350-3. [Crossref] [PubMed]
- Stachura A, Król R, Poplawski T, et al. Transfusion of intra-operative autologous whole blood: influence on complement activation and interleukin formation. Vox Sang 2011;100:239-46. [Crossref] [PubMed]
- Rollins KE, Trim NL, Luddington RJ, et al. Coagulopathy associated with massive cell salvage transfusion following aortic surgery. Perfusion 2012;27:30-3. [Crossref] [PubMed]
- Waters JH. Intraoperative blood recovery. ASAIO J 2013;59:11-7. [Crossref] [PubMed]
- Buys WF, Buys M, Levin AI. Reinfusate Heparin Concentrations Produced by Two Autotransfusion Systems. J Cardiothorac Vasc Anesth 2017;31:90-8. [Crossref] [PubMed]
- Ashworth A, Klein AA. Cell salvage as part of a blood conservation strategy in anaesthesia. Br J Anaesth 2010;105:401-16. [Crossref] [PubMed]
- Murray D. Acute normovolemic hemodilution. Eur Spine J 2004;13:S72-5. [Crossref] [PubMed]
- Lisander B. Preoperative haemodilution. Acta Anaesthesiol Scand Suppl 1988;89:63-70. [Crossref] [PubMed]
- Viele MK, Weiskopf RB. What can we learn about the need for transfusion from patients who refuse blood? The experience with Jehovah's Witnesses. Transfusion 1994;34:396-401. [Crossref] [PubMed]
- Barile L, Fominskiy E, Di Tomasso N, et al. Acute Normovolemic Hemodilution Reduces Allogeneic Red Blood Cell Transfusion in Cardiac Surgery: A Systematic Review and Meta-analysis of Randomized Trials. Anesth Analg 2017;124:743-52. [Crossref] [PubMed]
- Shander A, Brown J, Licker M, et al. Standards and Best Practice for Acute Normovolemic Hemodilution: Evidence-based Consensus Recommendations. J Cardiothorac Vasc Anesth 2020;34:1755-60. [Crossref] [PubMed]
- Ni Y, Xu ZJ, Zhang ZF, et al. Acute normovolemic hemodilution for major cancer surgeries during the COVID-19 pandemic: A beacon of hope. J Clin Anesth 2020;65:109871. [Crossref] [PubMed]
- Eroglu A, Uzunlar H, Erciyes N. Comparison of hypotensive epidural anesthesia and hypotensive total intravenous anesthesia on intraoperative blood loss during total hip replacement. J Clin Anesth 2005;17:420-5. [Crossref] [PubMed]
- Barak M, Yoav L, Abu el-Naaj I. Hypotensive anesthesia versus normotensive anesthesia during major maxillofacial surgery: a review of the literature. ScientificWorldJournal 2015;2015:480728. [Crossref] [PubMed]
- Gupta KK, Kumari V, Kaur S, et al. Comparative evaluation of propofol versus dexmedetomidine infusion for hypotensive anesthesia during functional endoscopic sinus surgery: a prospective randomized trial. Anesth Pain Med (Seoul) 2022;17:271-9. [Crossref] [PubMed]
- Juelsgaard P, Møller MB, Larsen UT. Preoperative Acute Normovolaemic Hemodilution (ANH) in combination with Hypotensive Epidural Anaesthesia (HEA) during knee arthroplasty surgery. No effect on transfusion rate. A randomized controlled trial BMC Anesthesiol 2002;2:1. [ISRCTN87597684]. [Crossref] [PubMed]
- Sharrock NE, Salvati EA. Hypotensive epidural anesthesia for total hip arthroplasty: a review. Acta Orthop Scand 1996;67:91-107. [Crossref] [PubMed]
- Lieberman JR, Huo MM, Hanway J, et al. The prevalence of deep venous thrombosis after total hip arthroplasty with hypotensive epidural anesthesia. J Bone Joint Surg Am 1994;76:341-8. [Crossref] [PubMed]
- Alcelik I, Pollock RD, Sukeik M, et al. A comparison of outcomes with and without a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty 2012;27:331-40. [Crossref] [PubMed]
- Hill SE, Broomer B, Stover J, et al. Bipolar tissue sealant device decreases hemoglobin loss in multilevel spine surgery. Transfusion 2012;52:2594-9. [Crossref] [PubMed]
- Fujita H, Iida H, Shimizu K, et al. A novel femoral intramedullary plug with sliding mechanism. J Arthroplasty 2003;18:367-70. [Crossref] [PubMed]
- Cui GY, Han XG, Wei Y, et al. Robot-Assisted Minimally Invasive Transforaminal Lumbar Interbody Fusion in the Treatment of Lumbar Spondylolisthesis. Orthop Surg 2021;13:1960-8. [Crossref] [PubMed]
- Pietsch M, Djahani O, Zweiger Ch, et al. Custom-fit minimally invasive total knee arthroplasty: effect on blood loss and early clinical outcomes. Knee Surg Sports Traumatol Arthrosc 2013;21:2234-40. [Crossref] [PubMed]
- Carvalho MV, Marchi E. Mechanism of action of topical hemostatic and adhesive tissue agents. Rev Med Minas Gerais 2013;23:488-93. [Crossref]
- Kietaibl AT, Kietaibl S. New Hemostatic Agents: Perioperative Anesthetic Considerations. Curr Pharm Des 2019;25:2158-64. [Crossref] [PubMed]
- Cullifer RM, Makai G, Pacis M, et al. Topical hemostatic and tissue-sealing agents in gynecologic surgery. Curr Opin Obstet Gynecol 2020;32:285-91. [Crossref] [PubMed]
- Giangrande PL, Wilde JT, Madan B, et al. Consensus protocol for the use of recombinant activated factor VII [eptacog alfa (activated); NovoSeven] in elective orthopaedic surgery in haemophilic patients with inhibitors. Haemophilia 2009;15:501-8. [Crossref] [PubMed]
- Guzzetta NA, Russell IA, Williams GD. Review of the off-label use of recombinant activated factor VII in pediatric cardiac surgery patients. Anesth Analg 2012;115:364-78. [Crossref] [PubMed]
- Zatta A, Mcquilten Z, Kandane-Rathnayake R, et al. The Australian and New Zealand Haemostasis Registry: ten years of data on off-licence use of recombinant activated factor VII. Blood Transfus 2015;13:86-99. [PubMed]
- Ortmann E, Besser MW, Klein AA. Antifibrinolytic agents in current anaesthetic practice. Br J Anaesth 2013;111:549-63. [Crossref] [PubMed]
- Diamond PT. Severe anaemia: implications for functional recovery during rehabilitation. Disabil Rehabil 2000;22:574-6. [Crossref] [PubMed]
- Gutsche JT, Kornfield ZN, Speck RM, et al. Impact of guideline implementation on transfusion practices in a surgical intensive care unit. J Cardiothorac Vasc Anesth 2013;27:1189-93. [Crossref] [PubMed]
- Shander A. Preoperative anemia and its management. Transfus Apher Sci 2014;50:13-5. [Crossref] [PubMed]
- Steuber TD, Howard ML, Nisly SA. Strategies for the Management of Postoperative Anemia in Elective Orthopedic Surgery. Ann Pharmacother 2016;50:578-85. [Crossref] [PubMed]
- Available online: https://www.who.int/publications/i/item/9789240033733
- Laso-Morales MJ, Vives R, Gómez-Ramírez S, et al. Intravenous iron administration for post-operative anaemia management after colorectal cancer surgery in clinical practice: a single-centre, retrospective study. Blood Transfus 2018;16:338-42. [PubMed]
- Muñoz M, Franchini M, Liumbruno GM. The post-operative management of anaemia: more efforts are needed. Blood Transfus 2018;16:324-5. [PubMed]
- Sultan P, Bampoe S, Shah R, et al. Oral vs intravenous iron therapy for postpartum anemia: a systematic review and meta-analysis. Am J Obstet Gynecol 2019;221:19-29.e3. [Crossref] [PubMed]
- Available online: https://www.caasn.com/protein-deficiency-anemia.html (assessed 12 Aug 22).
- Hertfelder HJ, Bös M, Weber D, et al. Perioperative monitoring of primary and secondary hemostasis in coronary artery bypass grafting. Semin Thromb Hemost 2005;31:426-40. [Crossref] [PubMed]
- Besser MW. Post-operative of bleeding, haemolysis and coagulation in mechanical circulatory support patients. Ann Transl Med 2020;8:832. [Crossref] [PubMed]
- Liumbruno GM, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion management of patients in the peri-operative period. III. The post-operative period. Blood Transfus 2011;9:320-35. [PubMed]
- del Junco DJ, Holcomb JB, Fox EE, et al. Resuscitate early with plasma and platelets or balance blood products gradually: findings from the PROMMTT study. J Trauma Acute Care Surg 2013;75:S24-30. [Crossref] [PubMed]
- Muñoz M, Acheson AG, Bisbe E, et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 2018;73:1418-31. [Crossref] [PubMed]
- Hedges SJ, Dehoney SB, Hooper JS, et al. Evidence-based treatment recommendations for uremic bleeding. Nat Clin Pract Nephrol 2007;3:138-53. [Crossref] [PubMed]
- Boccardo P, Remuzzi G, Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost 2004;30:579-89. [Crossref] [PubMed]
- Galbusera M, Remuzzi G, Boccardo P. Treatment of bleeding in dialysis patients. Semin Dial 2009;22:279-86. [Crossref] [PubMed]
- Banerjee S, Kapadia BH, Issa K, et al. Postoperative blood loss prevention in total knee arthroplasty. J Knee Surg 2013;26:395-400. [Crossref] [PubMed]
- Meyer HM, Torborg A, Cronje L, et al. The association between preoperative anemia and postoperative morbidity in pediatric surgical patients: A secondary analysis of a prospective observational cohort study. Paediatr Anaesth 2020;30:759-65. [Crossref] [PubMed]
- Charuvila S, Davidson SE, Thachil J, et al. Surgical decision making around paediatric preoperative anaemia in low-income and middle-income countries. Lancet Child Adolesc Health 2019;3:814-21. [Crossref] [PubMed]
- Goobie SM, Faraoni D, Zurakowski D, et al. Association of Preoperative Anemia With Postoperative Mortality in Neonates. JAMA Pediatr 2016;170:855-62. [Crossref] [PubMed]
- Partridge J, Harari D, Gossage J, et al. Anaemia in the older surgical patient: a review of prevalence, causes, implications and management. J R Soc Med 2013;106:269-77. [Crossref] [PubMed]
- Kifle E, Hussein M, Alemu J, et al. Prevalence of Anemia and Associated Factors among Newly Diagnosed Patients with Solid Malignancy at Tikur Anbessa Specialized Hospital, Radiotherapy Center, Addis Ababa, Ethiopia. Adv Hematol 2019;2019:8279789. [Crossref] [PubMed]
- Luan H, Ye F, Wu L, et al. Perioperative blood transfusion adversely affects prognosis after resection of lung cancer: a systematic review and a meta-analysis. BMC Surg 2014;14:34. [Crossref] [PubMed]
- Bohlius J, Bohlke K, Castelli R, et al. Management of cancer-associated anemia with erythropoiesis-stimulating agents: ASCO/ASH clinical practice guideline update. Blood Adv 2019;3:1197-210. [Crossref] [PubMed]
- Tiotiu A, Clerc-Urmès I, Martinet Y. Efficiency of erythropoiesis-stimulating agents (ESA) in the treatment of chemotherapy-induced anemia in patients with lung cancer (LC). European Respiratory Journal 2015 46: PA4842.
- Carson JL, Noveck H, Berlin JA, et al. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion 2002;42:812-8. [Crossref] [PubMed]
- Shander A, Goodnough LT. Management of anemia in patients who decline blood transfusion. Am J Hematol 2018;93:1183-91. [Crossref] [PubMed]
- Chand NK, Subramanya HB, Rao GV. Management of patients who refuse blood transfusion. Indian J Anaesth 2014;58:658-64. [Crossref] [PubMed]
- Hofmann A, Shander A, Blumberg N, et al. Patient Blood Management: Improving Outcomes for Millions While Saving Billions. What Is Holding It Up? Anesth Analg 2022;135:511-23. [Crossref] [PubMed]
- Rahav Koren R, Suriu C, Yakir O, et al. Physicians' lack of knowledge - a possible reason for red blood cell transfusion overuse? Isr J Health Policy Res 2017;6:49. [Crossref] [PubMed]
- Saporito A, La Regina D, Hofmann A, et al. Perioperative inappropriate red blood cell transfusions significantly increase total costs in elective surgical patients, representing an important economic burden for hospitals. Front Med (Lausanne) 2022;9:956128. [Crossref] [PubMed]
- The Joint Commission and the American Medical Association Convened Physician Consortium for Performance Improvement (PCPI) 2012. Available online: http://www.jointcommission.org/assets/1/6/National_Summit_Overuse.pdf (Accessed 15 Apr 2014).
- The urgent need to implement patient blood management: policy brief. World Health Organization. 2021. Available online: https://apps.who.int/iris/handle/10665/346655 (Accessed December 1, 2021)
- Hofmann A, Spahn DR, Holtorf AP, et al. Making patient blood management the new norm(al) as experienced by implementors in diverse countries. BMC Health Serv Res 2021;21:634. [Crossref] [PubMed]
- Hofmann A, Aapro M, Fedorova TA, et al. Patient blood management in oncology in the Russian Federation: Resolution to improve oncology care. J Cancer Policy 2022;31:100315. [Crossref] [PubMed]
- Hussey P, Onodera Y, Reddy S, et al. Need for preoperative anemia management clinics in Japan: initiatives at a university hospital in the USA. J Anesth 2021;35:710-22. [Crossref] [PubMed]
- Lucas J, Costa E, Subtil A, et al. Clinical, economical and safety impact of ferric carboxymaltose use in Patient Blood Management programme in Portuguese National Health Service hospitals. Sci Rep 2022;12:19335. [Crossref] [PubMed]
- Drabinski T, Zacharowski K, Meybohm P, et al. Estimating the Epidemiological and Economic Impact of Implementing Preoperative Anaemia Measures in the German Healthcare System: The Health Economic Footprint of Patient Blood Management. Adv Ther 2020;37:3515-36. [Crossref] [PubMed]
- Wyssusek K, Taylor K, Concha-Blamey S. Focused educational intervention improves intern knowledge of perioperative patient blood management principles. BMJ Open Qual 2021;10:e001390. [Crossref] [PubMed]
- Rambiritch V, Verburgh E, Louw VJ. Patient blood management and blood conservation - Complimentary concepts and solutions for blood establishments and clinical services in South Africa and beyond. Transfus Apher Sci 2021;60:103207. [Crossref] [PubMed]
- Molina-Arrebola MA, Fernández-Guerrero E, Aguirre-Ortega FJ, et al. Digital resources for transfusion education. J Educ Health Promot 2020;9:173. [Crossref] [PubMed]
- Hönemann C, Hagemann O, Doll D, et al. Reticulocyte hemoglobin equivalent as a diagnostic marker for the current iron deficiency: Old wine in new bottles. Anaesthesist 2020;69:919-25. [PubMed]
- Cappellini MD, Musallam KM, Taher AT. Iron deficiency anaemia revisited. J Intern Med 2020;287:153-70. [Crossref] [PubMed]
- Watson P, Watson D, Dhesi A, et al. Improving blood-prescribing decisions: Evaluating the efficacy of a blood guidelines app. Transfus Med 2020;30:485-91. [Crossref] [PubMed]
Cite this article as: Mandal S, Smith DL, Peter PJ, Louw VJ, Sil S, Ibrahim IN, Maji M, Nath S. Perioperative anaemia management. Ann Blood 2023;8:30.


