
Congenital thrombotic thrombocytopenic purpura: genetics and emerging therapies
The history and features of congenital thrombotic thrombocytopenic purpura (cTTP)
Thrombotic thrombocytopenic purpura (TTP) is a rare thrombotic disease caused by the severe depletion of ADAMTS13, which cleaves hemostatic glycoprotein and ultra-large von Willebrand factor (UL-VWF) multimers into a smaller quiescent state (1-3). The incidence of TTP is estimated to be 2–6 per million persons, and cTTP accounts for less than 5% of all TTP cases (4). In 1924, Dr. Eli Moschcowitz first reported the case of a 16-year-old girl who died after presenting with high fever, petechiae, hemisphere paralysis, and coma. Autopsy findings indicated multiple hyaline microthrombi in the heart, kidney, and spleen (5). The clinical presentation and outcome of 271 TTP patients were analyzed, suggesting that TTP is an acquired thrombotic disease (6). Meanwhile, four TTP cases in seven siblings were first reported in 1975, indicating that a part of TTP might be inherited via autosomal recessive inheritance (7). Several studies have established the features of TTP and its effective treatments. Plasma infusion could successfully treat some TTP patients in acute episodes; however, these patients developed frequent recurrence of TTP episodes called “chronic relapsing TTP” (8-11). In 1982, UL-VWF multimers were identified in patients with chronic relapsing TTP, suggesting that genetic factors could suppress an unknown but essential enzyme regulating VWF function in these patients (12). The enzyme was identified as VWF-cleaving proteinase (VWF-CP) in 1996 by two research groups (13,14), and both amino acids and DNA sequences demonstrated that VWF-CP was identified as ADAMTS13 (15-19). Finally, biallelic ADAMTS13 mutations were identified as the cause of severe congenital ADAMTS13 deficiency in cTTP.
ADAMTS13 can process highly active UL-VWF multimers into smaller, less reactive ones in healthy individuals. ADAMTS13 can circulate in a closed conformation in which the spacer and CUB domains interact (20-22). However, once the CUB domains bind to the D4 domain of VWF, ADAMTS13 is stretched into an open conformation (23) and multiple exosites within the protease, disintegrin, cysteine-rich, and spacer domains interact with complementary sites in the unfolded VWF A2 domain in a “molecular zipper” manner (22,24). Subsequently, the metalloprotease domain is activated to cleave the VWF A2 domain between Tyr1605 and Met1606 (25-27). In the absence of functional ADAMTS13, owing to ADAMTS13 mutations, stretched UL-VWF multimers are prone to bind to circulating platelets and form abnormal VWF/platelet-rich microthrombi, which are mostly located in the arterioles and capillaries of the brain, heart, and kidneys, where they cause ischemic organ damage with a potentially fatal outcome.
cTTP is diagnosed using the following criteria: the presence of severe thrombocytopenia and Coombs-negative hemolytic anemia of unknown etiology, severe depletion of ADAMTS13 activity below 10% of healthy individuals, absence of anti-ADAMTS13 antibodies (anti-ADAMTS13 IgG autoantibodies or anti-ADAMTS13 inhibitors), and presence of biallelic ADAMTS13 mutations in homozygous or compound heterozygous mode (28,29). ADAMTS13 activity is measured mainly by two different assays: a fluorescence resonance energy transfer (FRET) assay using FRETS-VWF73 as a substrate (30) and a chromogenic ADAMTS13 activity ELISA using a murine monoclonal antibody that specifically recognizes Y1605 of the VWF cleavage site (31). Notably, the FRET-VWF73 assay sometimes fails to determine the ADMATS13 activity in patients with hyperbilirubinemia [>100 µmol/L (5.85 mg/dL)] because high levels of bilirubin interfere with fluorescence evolution by acting as a quencher at an emission wavelength of 450 nm (32). Although all cTTP patients show severely decreased ADAMTS13 activity over time, they may show a normal range of platelet counts, lactate dehydrogenase (LDH), and other TTP symptoms in the absence of TTP triggers. We believe that more potential cTTP patients are left undiagnosed or misdiagnosed with immune-mediated TTP (iTTP) in childhood, ABO/Rh-incompatibility in newborns, or Hemolysis Elevated Liver enzymes and Low Platelets (HELLP) syndrome in pregnancy. Therefore, clinicians must recognize that cTTP cannot be diagnosed by clinical presentation, such as the pentad of clinical symptoms no longer used in clinical practice without the ADAMTS13 test. As for anti-ADAMTS13 antibodies (IgG autoantibodies or anti-ADAMTS13 inhibitors), we sometimes encounter cases difficult to diagnose as either cTTP or iTTP, which show a negative or borderline detection limit. In these cases, patients can only be diagnosed after monitoring their ADAMTS13 activity and analyzing ADAMTS13 activity in their parents because a mild to moderate depletion of ADAMTS13 activity is consistently seen (20–50%).
Since few review papers have clearly described the characteristics, epidemiology, long-term outcomes, and ideal treatment for patients with cTTP, physicians obtain an inadequate amount of information. Hence, we attempted to provide an overview of cTTP and reveal the significant advances and controversial points, focusing on genetics and emerging therapies in cTTP.
National cTTP cohorts and patients’ associations
As the prevalence of cTTP has been estimated at approximately one in every one million persons (33-36), it is difficult to clarify the links between genotype and clinical presentation, optimal treatment regimen, and long-term outcomes. Large-scale cTTP cohorts have been found in several countries, including the United Kingdom (37), Switzerland (international hereditary TTP registry) (38), France (39), and Japan (40), which have progressively demonstrated robust evidence over the past few decades. Many patients and their parents feel anxious regarding long-term treatment and upcoming TTP attacks because their physicians provide limited information regarding treatment and long-term outcomes of this extremely rare disease. As with other rare diseases, patient groups can enhance the exchange of experiences and information among patients and their families. The Japanese group for cTTP patients has been promoting these activities via regular online meetings (https://cttpjapan2020.wixsite.com/adamts13).
ADAMTS13 mutations in cTTP patients
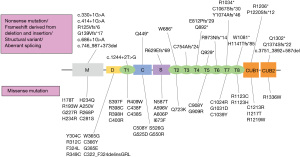
ADAMTS13 is found on chromosome 9q34 and encodes ADAMTS13 protein of 1427 amino acids (15,17). After identifying ADAMTS13 causative mutations in 2001, more than 200 different causative mutations have been reported worldwide (33). These mutations are inherited in an autosomal recessive manner from parents to siblings, and even one causative mutation reduces ADAMTS13 activity by 20–50% (28). In contrast, de novo ADAMTS13 causative mutations were also reported from the Japanese cTTP cohort and other groups (41-43). In all mutations, missense mutations are the most dominant (approximately half of the cases), followed by nonsense, frameshift mutations derived from deletion or insertion, and splice-site mutations (37,38,40). ADAMTS13 mutations are spread across all ADAMTS13 domains with no accumulated gene hotspots, suggesting that various causative mutations impair ADAMTS13 activity in different ways.
Protein expression studies using cultured mammalian cells revealed that although some ADAMTS13 mutated proteins were secreted, most mutated proteins were retained in the cell because of interrupted protein secretion (44,45). Several in vitro experiments have demonstrated that ADAMTS13 undergoes various types of glycosylation as a post-translational modification (46), and incomplete glycosylation leads to the disruption of secretion of this enzyme, reduced enzyme activity, or clearance in circulation. Based on mass spectral analysis of tryptic peptides derived from ADAMTS13, at least six thrombospondin type 1 repeat (TSP) domains were modified with an O-fucose disaccharide. ADAMTS13 secretion was substantially inhibited when the gene expression of O-fucosyltransferase 2, an enzyme that transfers fucose to serine in the TSP domain, was interrupted by siRNA in HEK293 T-REx cells (47). Other research groups also found that N-linked glycans modulate ADAMTS13 secretion and VWF cleavage activity (48,49). C-mannosylation sites were identified in TSP1, linker TSP4-TSP5, and TSP8, which also play an essential role in the secretion of TSP-containing proteins (50-52). In summary, ADAMTS13 mutations cause structural abnormalities or sometimes disrupt post-translational modifications, leading to a severe impairment of ADAMTS13 activity.
Here, we present a summary of genetic information in the Japanese cTTP cohort. As of May 2022, 68 patients were clinically diagnosed with cTTP in the registry, and 67 patients underwent ADAMTS13 analysis using Sanger’s method. One patient did not undergo the ADAMTS13 analysis. When causative mutations were not detected, comprehensive genomic quantitative polymerase chain reaction (qPCR) analysis was performed to complement the weakness of PCR direct sequencing (53). A pair of causative ADAMTS13 mutations was identified in 63 of the 67 patients who underwent ADAMTS13 analysis. In total, 68 different mutations were identified in the 60 families (Figure 1). Although most of these were unique, there were two predominant mutations (p.R193W and p.C908Y) in the Japanese cohort (Figure 2). p.R193W is located in the metalloprotease domain, whereas p.C908Y occurs immediately after the TSP4-5 linker region, suggesting that p.R193W may directly affect the catalytic activity of expressed ADAMTS13, and p.C908Y can deteriorate the flexibility of the linker region, leading to conformational abnormality (54). These dominant mutations are inconsistent with those reported in the European populations, in which c.4143_4144dupA (p.E1382Rfs*6) and p.R1060W were the prevalent ADAMTS13 mutations (37,38). These European mutations were not identified in the Japanese cohort. Notably, although the prevalent ADAMTS13 mutations differed between Caucasian and Japanese patients, a previous study reported that they may share similar clinical presentations (38).
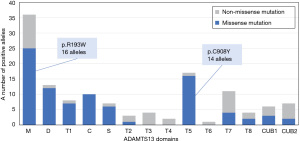
The link between genotype and phenotype has been discussed for over two decades. In Europe, the frequent mutation, p.R1060W, was found to be associated with low residual ADAMTS13 activity (5–10% in homozygotes of healthy individuals) and was well determined in primigravida patients (37,38,55,56). In a Japanese cohort, two prevalent mutations, p.R193W and p.C908Y, were found to not be associated with clinical presentation, including fresh frozen plasma (FFP) dependency, survival rate, and long-term organ damage. When focusing on the combination of mutation sites and FFP dependency, no patient in the on-demand FFP infusion group had cysteine-rich, TSP3, TSP6, or CUB2 domain mutations. In addition, homozygous mutations in the cysteine-rich, TSP3, TSP5, and TSP7 domains were present only in the prophylactic FFP infusion group (57). Furthermore, cTTP siblings with the same pair of ADAMTS13 mutations do not always show the same clinical presentation; for example, although one patient required prophylactic FFP infusion to maintain platelet counts within the normal range and prevent TTP episodes, the other did not develop progressive thrombocytopenia even without FFP infusion for long periods. This phenomenon suggests that cTTP is caused by biallelic ADAMTS13 mutations, and multifactorial triggers evoke TTP episodes (38).
The efficacy and limitations of current plasma therapy regimens
The efficacy of plasma therapy has been described in patients with chronically relapsing TTP (8-11). Meanwhile, iTTP patients require extensive plasma exchange and immunosuppressors for ADAMTS13 supplementation and for the removal of autoantibodies and UL-VWF multimers. One or two units of plasma infusion quickly achieve complete improvement in platelet counts and LDH levels among cTTP patients in the acute phase (1). Platelet counts can be maintained within the normal range for 2 weeks, whereas ADAMTS13 activity returns to baseline 2.5–3.5 days after plasma infusion (58-60). The recent International Society of Thrombosis and Hemostasis (ISTH) TTP guidelines recommend treating cTTP with plasma infusion (10 to 15 mL/kg) every 1 to 3 weeks as maintenance therapy and daily plasma infusion for symptomatic patients until the symptoms resolve and platelet counts normalize, as previously described (29,33,61,62). Similarly, 240–480 mL of prophylactic plasma infusion every 2 weeks has been performed over the past two decades in Japan (63). To date, the optimal starting timing of FFP and dosage/intervals remains open to discussion. When we investigated the issues for prophylactic plasma infusion, most patients and their families complained about allergic reactions, such as urticaria during infusion, and in the worst case, life-threatening anaphylaxis induced by plasma-derived protein. Plasma products can transmit pathogenic organisms from each blood donor, and three patients are infected with hepatitis C virus via infused plasma (57).
In addition to normal FFP, the efficacy of two plasma-derived factor VIII/VWF concentrates (Koate-DVI and BPL 8Y) has also been reported by a UK group (64,65). As the volume of these agents is smaller than that of a plasma bag, these options can benefit small children or patients who require desensitization because of fatal hypersensitivity to plasma. Regarding hypersensitivity against plasma, solvent-detergent inactivated and amotosalen-UVA pathogen inactivated plasmas reduce severe allergic reactions (66-68). However, these agents are unavailable in some countries, including Japan.
In contrast to hemophilia patients receiving prophylactic treatments, the development of ADAMTS13 autoantibodies among cTTP patients has not been frequently argued. A few cases involving inhibitory and non-inhibitory ADAMTS13 autoantibodies have shown that these antibodies did not lead to resistance to plasma infusion or increased clearance of ADAMTS13 (2,40,69). The possible hypothesis for this rare autoantibody production is based on the interaction between spacer and CUB domains takes a looped conformation (closed ADAMTS13) and hinders the essential cryptic epitope from the molecular surface (20-22). The following recombinant human (rh)ADAMTS13 studies will further shed light on this matter.
Special attention to children and pregnant females
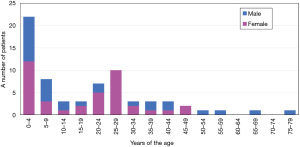
When prophylactic FFP infusion is durable in terms of physical burden (frequent hospital visits) and allergic adverse events, severe thrombocytopenia and fatal thrombotic events may be avoidable. However, some patients can maintain platelet counts and LDH levels within the normal range despite sustaining severe ADAMTS13 depletion (activity level, <0.5%) and are often followed up without prophylactic plasma infusion (57). Moreover, it remains yet unknown whether asymptomatic patients should also receive plasma therapy in their lifetime. However, a recent study revealed that the annual incidence of acute episodes was the highest in patients below 10 years of age and decreased with age. This result suggests that these patients are generally susceptible to many viral infections during childhood, followed by repeated TTP episodes. In addition, as we previously reported, two-thirds of patients developed severe neonatal jaundice in combination with thrombocytopenia on the day of birth, leading to the diagnosis of cTTP (Figure 3) (29,38,40,70). Therefore, pediatric patients should receive prophylactic ADAMTS13 supplements regardless of the disease activity. However, current plasma therapies cannot overcome these problems, including physical burden (frequent hospital visits) and allergic adverse events. In Japan, prophylactic FFP infusion is usually recommended for all cTTP patients; however, most infants or pediatric patients are monitored by close follow-up and treated with on-demand FFP infusion in the case of TTP episodes until reaching adolescence (57).
Pregnancy is also a well-known trigger for acute episodes in female cTTP patients, and both mothers (cTTP patients) and babies are at risk of fatal thrombosis (33,55,56,71-74). Our recent study showed that intrauterine fetal death occurred in up to 50% of the cases unless pregnant patients received prophylactic FFP infusion. The immature placenta with no nuclei in the villi and some inflammatory cells in the intervillous space suggests microinfarction in the placenta (73,75). Even normal pregnancies in healthy individuals after second semester showed higher levels of VWF of more than 200–400% (76,77). An imbalance between increased VWF antigen levels and sustained ADAMTS13 depletion would fail to maintain a suitable environment for the mother and fetus. Hence, we should discuss the optimal therapeutic regimen for cTTP pregnancy. Although miscarriage is commonly seen in the first semester (8–12 weeks of gestation) among healthy individuals, fetal survival rates markedly decreased after 20 weeks of gestation in cTTP patients without FFP infusion. In addition, the VWF antigen level in cTTP patients is markedly increased after 35–36 weeks of gestation, even with intense FFP replenishment. Intriguingly, we observed that one patient with detectable ADAMTS13 activity (±5%) successfully delivered her baby twice without any thrombotic event for the mother or growing restriction of her baby, despite not receiving prophylactic FFP infusion during pregnancy. Based on these results and experiences, we propose a therapeutic regimen for safer delivery as follows: closed monitoring of ADAMTS13 activity, platelet counts, LDH levels, and fetal growth throughout pregnancy; if a patient does not receive prophylactic FFP infusion after diagnosis (on-demand FFP infusion), she should start a weekly FFP supply for at least 20 weeks of gestation; an FFP infusion dosage of at least 5 mL/kg/week to maintain pregnancy; and planned delivery at 36–38 weeks to avoid unfavorable thrombotic events in both the mother and baby. We speculate that the optimal ADAMTS13 activity trough is more than 5% during pregnancy. However, it is difficult to set a definite threshold for ADAMTS13 activity trough because of its clinically heterogeneous presentation. The risk factors for fatal outcomes are considered recurrent TTP episodes before conception and additional VWF boosting by infection and alcohol abuse. We also must provide female patients and their parents with information that pregnancy poses a risk for cTTP and they will also be supposed to have TTP episodes during the upcoming pregnancy without close follow-up for ADAMTS13 activity and extensive ADAMTS13 replenishment.
cTTP: Long-term survival rate and complications
Long-term survival rates and complications in cTTP that impair the quality of life of patients remain unelucidated. The accumulated data in the Japanese cTTP cohort from 1998 revealed the overall survival, cause of death, and long-term mortality. As of June 2022, 10 of the 68 patients died within the follow-up periods. Figure 4 shows that the 10-year overall survival rate was 91.1%, and the median age at death, except for one suicide case, was 44 (IQR: 41–52). Many patients who died experienced end-stage renal failure, requiring renal replacement therapy and suffered from sudden death. Myocardial infarction, heart failure, and arrhythmia have been described as the causes of sudden death (78,79). The causes of death in our cTTP cohort are summarized in Table 1. Previous studies have revealed that increased VWF antigen levels lead to high mortality risk in dialysis patients without cTTP (80,81). In addition, dialysis patients with high VWF antigen levels and low ADAMTS13 activity have a 2.2-fold higher mortality rate than those with low VWF antigen levels and high ADAMTS13 activity (82). Up to one-fourth of hemodialysis patients died of sudden cardiac death, which might have resulted from the fact that hemodialysis is associated with both ventricular arrhythmia and dynamic electrocardiographic changes (83,84). Histopathological findings of renal biopsy from cTTP patients with progressive renal impairment revealed chronic glomerular sclerotic changes and more C4d deposits than those in non-cTTP cases, suggesting that C4d immunostaining provides evidence for complement-mediated glomerular damage in cTTP patients (85). Our long-term follow-up study investigated 55 cTTP patients also indicated that 41 patients with prophylactic FFP were considered to have a more severe form of the disease, lower platelet counts, and higher serum creatinine levels than 14 patients receiving on-demand FFP and developed more organ damage, including renal impairment, cerebral infarction, and myocardial infarction during follow-up periods. Two deceased cases were in the prophylactic FFP group. Intriguingly, no patient in the on-demand FFP group showed organ damage during the follow-up period. The FFP-dependent group received 13.2 mL/kg of FFP infusion per month (median), lower than the ISTH guideline (20.0–30.0 mL/kg per month) (57). In addition, a very recent interview study of 26 patients in the United States revealed that all patients, except one infant, suffered from more than two neuropsychiatric symptoms, namely, headaches, poor concentration, and depression. Other symptoms included headaches with aura (presumed migraine), vision changes, forgetfulness, fatigue, neuropathy, dysarthria, loss of vision, seizures, transient weakness, falls, and dysphagia (86). This study also found that 17 (63%) patients suffered from strokes as they aged. Eleven of the 17 patients had stroke-related disabilities. The high incidence of stroke among cTTP patients is similar to that in our previous study (57). Notably, the reduction of ADAMTS13 activity in patients with iTTP during the remission phase led to an increased risk of stroke (87).
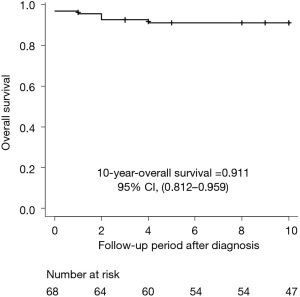
Table 1
| Code | Age of death, year | Sex | Follow-up year | Cause of death | Complications | Prophylactic FFP infusion |
|---|---|---|---|---|---|---|
| C3 | 38 | M | 30 | Unknown* | ESRD (HD) | YES† |
| H3 | 52 | M | 1 | Uremia | ESRD (HD), GIH | NO |
| R5 | 37 | F | 14 | Suicide | YES (10.0 mL/kg/4 w) | |
| X5 | 44 | F | 4 | Unknown* | SLE | NO |
| 2G2 | 79 | M | 3 | Cerebral infarction | YES (24.1 mL/kg/4 w) | |
| 2N4 | 23 | F | 0 | Unknown* | Pregnancy | NO |
| 2O | 41 | M | 2 | Unknown* | ESRD (HD) | NO |
| 2P4 | 44 | F | 17 | Status epilepticus, NOMI | Paralysis after stroke | YES (17.0 mL/kg/4 w) |
| 2R | 48 | M | 14 | Unknown* | ESRD (HD) | YES (9.8 mL/kg/4 w) |
| 2T | 66 | M | 1 | Sepsis | ESRD (HD) | YES† |
*, patients experienced sudden death, suggesting sudden cardiac death; †, exact body weight data were not available for these patients. FFP, fresh frozen plasma; ESRD, end-stage renal disease; HD, hemodialysis; GIH, gastrointestinal hemorrhage; SLE, systemic lupus erythematosus; NOMI, non-occlusive mesenteric ischemia.
Taken together, we hypothesized that under insufficient ADAMTS13 supply, patients dependent on FFP infusion can be exposed to potential risks of systemic stealth microthrombi evoked by inflammatory reactions with unregulated complement activation (88). The international registry group also described that insufficient ADAMTS13 replenishment in patients leads to recurrent TTP episodes (89). Meanwhile, patients independent of prophylactic FFP infusion seem to exhibit a much better outcome than those who require it, even for long follow-up periods (57). Hence, these differences might depend on substantial complement activation, particularly in vascular regions with high endothelial shear stress as well as deficient ADAMTS13 expression.
Emerging new therapeutic agents for cTTP
Similar to any hereditary deficiency of coagulation factors, the concept of replenishing recombinant enzymes has been investigated even for cTTP patients. The rhADAMTS13 product (BAX930/SHP-655/TAK-755) was originally developed by Baxter (now integrated with Takeda) and completed the phase I first-in-human trial (NCT02216084) (90) and has been underway in phase III international multicenter study with two arms: the prophylaxis and the on-demand cohorts (NCT04683003 and NCT03393975, respectively). The protective effect of rhADAMTS13 was previously confirmed in a cTTP mouse model induced by recombinant VWF (rVWF) concentrate in Adamts13 knockout mice (KO) (91). The administration of rhADAMTS13 improves the clinical and hematological appearance of TTP episodes. Hence, the rhADAMTS13 product is a promising therapeutic option that could overcome several limitations of prophylactic FFP infusion. Furthermore, patients could be free from intolerant allergic reactions and easily receive intravenous injections at home. The rhADAMTS13 product enables patients to reach sufficient ADAMTS13 activity at a trough level, suggesting that we can establish a much safer therapeutic regimen not only for children and pregnant patients but also for preventing long-term ischemic organ damage (33,57,73). The first-in-human clinical trial was conducted to determine if the pharmacokinetic (PK) profile was comparable to that of FFP infusion studies, as well as to confirm the safety, immunogenicity, and tolerability among cTTP patients dependent on FFP infusion (90). The authors demonstrated that the PK profile of rhADAMTS13 was similar to that of plasma-derived ADAMTS13 contained in FFP, with dose proportionality regarding Cmax and AUC after single-dose infusion at three different doses (5, 20, and 40 U/kg). rhADAMTS13 was well tolerated in cTTP patients and did not lead to serious adverse events and trigger anti-ADAMTS13 alloantibody production. During phase III clinical trials, participants in the prophylaxis cohort will receive an intravenous infusion of 40 IU/kg of TAK-755 once every week or once biweekly for approximately three years and will be allowed to opt for treatment in a home setting by a caregiver or by self-infusion. Based on a previous trial, 40 IU/kg was calculated to adjust the amount of ADAMTS13 used for single-volume plasma exchange in iTTP (90). Within a few years, our therapeutic strategy for all cTTP patients will markedly change after the approval of rhADAMTS13 use in practice. However, we still need to identify the ideal target trough level of ADAMTS13 activity to prevent long-term comorbidities caused by accumulated microvascular thrombi.
Gene therapy might also overcome the limitations of ADAMTS13 replacement because ADAMTS13 trans-gene could cure cTTP by expressing intact ADAMTS13 protein. To date, several clinical trials for gene therapy have investigated both therapeutic protein expression and safety for monogenic inherited diseases, including hemophilia A and B (92,93), metachromatic leukodystrophy (94) and X-linked severe combined immunodeficiency disorder (95). In blood disorders, gene therapy for hemophilia has been extensively explored over the years, and several phase III clinical trials have been conducted. Similar to cTTP patients, those with hemophilia also want to be free from lifetime injections. Long-term patient follow-up will establish effective gene expression and prevent long-term adverse events, leading to a shift from factor replacement therapy to gene therapy. Although the huge cost of gene therapy has been debated, a recent study reported that in patients with hemophilia A, gene therapy (valoctocogene roxaparvovec) would be more cost-effective than prophylactic therapy (96). In the field of cTTP, several research groups have already developed gene therapy using a viral gene transfer approach (lentivirus or adenovirus vectors) in Adamts13 KO mice, resulting in the expression of functional ADAMTS13 or truncated MDTCS fragments (97-100). One study demonstrated that induced ADAMTS13/MDTCS fragments successfully prevented TTP attacks evoked by a specific trigger, the Shiga toxin (100). The Belgian group has developed a non-viral integrating Sleeping Beauty transposon system to obtain long-term expression of superphysiological levels of ADAMTS13 for more than 25 weeks and demonstrated preventive effects against TTP attacks triggered by rVWF concentrate even 20 weeks after gene transfer (101). However, more information regarding long-term safety, including low toxicity, no tumorigenesis, and no germline transmission, must be confirmed in preclinical studies using different Adamts13 KO mammals. Based on previous hemophilia studies, acute liver damage induced by a high amount of viral vector, the presence of anti-viral vector antibodies, and the possibility of repeated viral vector administration have been repeatedly debated (102).
However, gene therapy development for cTTP has been slow compared to that for other diseases, such as hemophilia, even though previous ADAMTS13 expression studies in Adamts13 KO mice showed no critical adverse events and long-term (more than two years) expression. This suggests that the ADMATS13 transgene in host cells (mostly hepatocytes) is possible because the ADAMTS13 protein is derived from a single gene and the length of the coding sequence (3.7 kb) is acceptable for gene therapy. However, many research groups have difficulties in promoting gene therapy for extremely rare diseases because of a lack of financial support from pharmacological companies. The situation is the same for cTTP. To overcome this issue, robust efforts to enhance the collaboration between international working groups and patient associations in advocating for gene therapy for cTTP is critical. Further preclinical and clinical studies in the future are also warranted. Patients with cTTP should be offered gene therapy and be free from replenishment treatment for extended periods in future.
Acknowledgments
The authors would like to thank Professor Emeritus Yoshihiro Fujimura for his contribution to the cTTP registry in Japan and all physicians for sending the data and samples of Japanese patients with cTTP.
Funding: This work was financially supported by research grants from the Ministry of Health, Labour, and Welfare of Japan (20FC1024 to K.K. and M.M.).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (X. Long Zheng) for the series “Thrombotic Thrombocytopenic Purpura” published in Annals of Blood. The article has undergone external peer review.
Peer Review File: Available at https://aob.amegroups.com/article/view/10.21037/aob-22-17/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-22-17/coif). The series “Thrombotic Thrombocytopenic Purpura” was commissioned by the editorial office without any funding or sponsorship. KS was granted by Takeda (Takeda Japan Medical Office Funded Research Grant 2021). KK is one of the inventors of an engineered substrate for ADAMTS13 activity, and the license fee was paid to the National Cardiovascular Center, where he is affiliated. MM is a member of the advisory board of Takeda Yakuhin and Sanofi. He is also an inventor of the ADAMTS13 act-ELISA. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sadler JE. Pathophysiology of thrombotic thrombocytopenic purpura. Blood 2017;130:1181-8. [Crossref] [PubMed]
- Kremer Hovinga JA, Coppo P, Lämmle B, et al. Thrombotic thrombocytopenic purpura. Nat Rev Dis Primers 2017;3:17020. [Crossref] [PubMed]
- Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood 2017;129:2836-46. [Crossref] [PubMed]
- Zheng XL, Vesely SK, Cataland SR, et al. ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura. J Thromb Haemost 2020;18:2486-95. [Crossref] [PubMed]
- Moschcowitz E. Hyaline thrombosis of the terminal arterioles and capillaries: A hitherto undescribed disease. Proceeding New York Pathological Society 1924;24:21-4.
- Amorosi EL, Ultmann JE. Thrombotic thrombocytopenic purpura: report of 16 cases and review of the literature. Medicine 1966;45:139-60. [Crossref]
- Wallace DC, Lovric A, Clubb JS, et al. Thrombotic thrombocytopenic purpura in four siblings. Am J Med 1975;58:724-34. [Crossref] [PubMed]
- Upshaw JD Jr. Congenital deficiency of a factor in normal plasma that reverses microangiopathic hemolysis and thrombocytopenia. N Engl J Med 1978;298:1350-2. [Crossref] [PubMed]
- SCHULMAN I. Studies on thrombopoiesis. I. A factor in normal human plasma required for platelet production; chronic thrombocytopenia due to its deficiency. Blood 1960;16:943-57. [Crossref] [PubMed]
- Byrnes JJ, Khurana M. Treatment of thrombotic thrombocytopenic purpura with plasma. N Engl J Med 1977;297:1386-9. [Crossref] [PubMed]
- Lian EC, Harkness DR, Byrnes JJ, et al. Presence of a platelet aggregating factor in the plasma of patients with thrombotic thrombocytopenic purpura (TTP) and its inhibition by normal plasma. Blood 1979;53:333-8. [Crossref] [PubMed]
- Moake JL, Rudy CK, Troll JH, et al. Unusually large plasma factor VIII:von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med 1982;307:1432-5. [Crossref] [PubMed]
- Furlan M, Robles R, Lämmle B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood 1996;87:4223-34. [Crossref] [PubMed]
- Tsai HM. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood 1996;87:4235-44. [Crossref] [PubMed]
- Fujikawa K, Suzuki H, McMullen B, et al. Purification of human von Willebrand factor-cleaving protease and its identification as a new member of the metalloproteinase family. Blood 2001;98:1662-6. [Crossref] [PubMed]
- Gerritsen HE, Robles R, Lämmle B, et al. Partial amino acid sequence of purified von Willebrand factor-cleaving protease. Blood 2001;98:1654-61. [Crossref] [PubMed]
- Levy GG, Nichols WC, Lian EC, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature 2001;413:488-94. [Crossref] [PubMed]
- Zheng X, Chung D, Takayama TK, et al. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem 2001;276:41059-63. [Crossref] [PubMed]
- Soejima K, Mimura N, Hirashima M, et al. A novel human metalloprotease synthesized in the liver and secreted into the blood: possibly, the von Willebrand factor-cleaving protease? J Biochem 2001;130:475-80. [Crossref] [PubMed]
- Muia J, Zhu J, Gupta G, et al. Allosteric activation of ADAMTS13 by von Willebrand factor. Proc Natl Acad Sci U S A 2014;111:18584-9. [Crossref] [PubMed]
- South K, Luken BM, Crawley JT, et al. Conformational activation of ADAMTS13. Proc Natl Acad Sci U S A 2014;111:18578-83. [Crossref] [PubMed]
- Petri A, Kim HJ, Xu Y, et al. Crystal structure and substrate-induced activation of ADAMTS13. Nat Commun 2019;10:3781. [Crossref] [PubMed]
- Zanardelli S, Chion AC, Groot E, et al. A novel binding site for ADAMTS13 constitutively exposed on the surface of globular VWF. Blood 2009;114:2819-28. [Crossref] [PubMed]
- Crawley JT, de Groot R, Xiang Y, et al. Unraveling the scissile bond: how ADAMTS13 recognizes and cleaves von Willebrand factor. Blood 2011;118:3212-21. [Crossref] [PubMed]
- Zheng X, Nishio K, Majerus EM, et al. Cleavage of von Willebrand factor requires the spacer domain of the metalloprotease ADAMTS13. J Biol Chem 2003;278:30136-41. [Crossref] [PubMed]
- Schelpe AS, Petri A, Roose E, et al. Antibodies that conformationally activate ADAMTS13 allosterically enhance metalloprotease domain function. Blood Adv 2020;4:1072-80. [Crossref] [PubMed]
- Siedlecki CA, Lestini BJ, Kottke-Marchant KK, et al. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood 1996;88:2939-50. [Crossref] [PubMed]
- Matsumoto M, Fujimura Y, Wada H, et al. Diagnostic and treatment guidelines for thrombotic thrombocytopenic purpura (TTP) 2017 in Japan. Int J Hematol 2017;106:3-15. [Crossref] [PubMed]
- Scully M, Hunt BJ, Benjamin S, et al. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol 2012;158:323-35. [Crossref] [PubMed]
- Kokame K, Nobe Y, Kokubo Y, et al. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol 2005;129:93-100. [Crossref] [PubMed]
- Kato S, Matsumoto M, Matsuyama T, et al. Novel monoclonal antibody-based enzyme immunoassay for determining plasma levels of ADAMTS13 activity. Transfusion 2006;46:1444-52. [Crossref] [PubMed]
- Meyer SC, Sulzer I, Lämmle B, et al. Hyperbilirubinemia interferes with ADAMTS-13 activity measurement by FRETS-VWF73 assay: diagnostic relevance in patients suffering from acute thrombotic microangiopathies. J Thromb Haemost 2007;5:866-7. [Crossref] [PubMed]
- Kremer Hovinga JA, George JN. Hereditary Thrombotic Thrombocytopenic Purpura. N Engl J Med 2019;381:1653-62. [Crossref] [PubMed]
- Fujimura Y, Matsumoto M. Registry of 919 patients with thrombotic microangiopathies across Japan: database of Nara Medical University during 1998-2008. Intern Med 2010;49:7-15. [Crossref] [PubMed]
- Scully M, Yarranton H, Liesner R, et al. Regional UK TTP registry: correlation with laboratory ADAMTS 13 analysis and clinical features. Br J Haematol 2008;142:819-26. [Crossref] [PubMed]
- Mariotte E, Azoulay E, Galicier L, et al. Epidemiology and pathophysiology of adulthood-onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): a cross-sectional analysis of the French national registry for thrombotic microangiopathy. Lancet Haematol 2016;3:e237-45. [Crossref] [PubMed]
- Alwan F, Vendramin C, Liesner R, et al. Characterization and treatment of congenital thrombotic thrombocytopenic purpura. Blood 2019;133:1644-51. [Crossref] [PubMed]
- van Dorland HA, Taleghani MM, Sakai K, et al. The International Hereditary Thrombotic Thrombocytopenic Purpura Registry: key findings at enrollment until 2017. Haematologica 2019;104:2107-15. [Crossref] [PubMed]
- Joly BS, Boisseau P, Roose E, et al. ADAMTS13 Gene Mutations Influence ADAMTS13 Conformation and Disease Age-Onset in the French Cohort of Upshaw-Schulman Syndrome. Thromb Haemost 2018;118:1902-17. [Crossref] [PubMed]
- Fujimura Y, Matsumoto M, Isonishi A, et al. Natural history of Upshaw-Schulman syndrome based on ADAMTS13 gene analysis in Japan. J Thromb Haemost 2011;9:283-301. [Crossref] [PubMed]
- Kokame K, Aoyama Y, Matsumoto M, et al. Inherited and de novo mutations of ADAMTS13 in a patient with Upshaw-Schulman syndrome. J Thromb Haemost 2008;6:213-5. [Crossref] [PubMed]
- Alharbi I, Alqarni S, Khayyat W, et al. De Novo Mutation of the ADAMTS13 Gene with Mesenteric Ischemia in an Infant with Congenital Thrombotic Thrombocytopenic Purpura. Case Rep Hematol 2021;2021:5516863. [Crossref] [PubMed]
- Lv H, Wang Z, Yang L, et al. Neonate with Congenital Thrombotic Thrombocytopenic Purpura: a Case Report of a de novo Compound Heterozygote Mutation in ADAMTS13 Gene and Review of Literature. Clin Lab 2020;66: [Crossref] [PubMed]
- Kokame K, Matsumoto M, Soejima K, et al. Mutations and common polymorphisms in ADAMTS13 gene responsible for von Willebrand factor-cleaving protease activity. Proc Natl Acad Sci U S A 2002;99:11902-7. [Crossref] [PubMed]
- Roose E, Tersteeg C, Demeersseman R, et al. Anti-ADAMTS13 Antibodies and a Novel Heterozygous p.R1177Q Mutation in a Case of Pregnancy-Onset Immune-Mediated Thrombotic Thrombocytopenic Purpura. TH Open 2018;2:e8-e15. [Crossref] [PubMed]
- Sorvillo N, Kaijen PH, Matsumoto M, et al. Identification of N-linked glycosylation and putative O-fucosylation, C-mannosylation sites in plasma derived ADAMTS13. J Thromb Haemost 2014;12:670-9. [Crossref] [PubMed]
- Ricketts LM, Dlugosz M, Luther KB, et al. O-fucosylation is required for ADAMTS13 secretion. J Biol Chem 2007;282:17014-23. [Crossref] [PubMed]
- Zhou W, Tsai HM. N-Glycans of ADAMTS13 modulate its secretion and von Willebrand factor cleaving activity. Blood 2009;113:929-35. [Crossref] [PubMed]
- Nowak AA, O'Brien HER, Henne P, et al. ADAMTS-13 glycans and conformation-dependent activity. J Thromb Haemost 2017;15:1155-66. [Crossref] [PubMed]
- Wang LW, Leonhard-Melief C, Haltiwanger RS, et al. Post-translational modification of thrombospondin type-1 repeats in ADAMTS-like 1/punctin-1 by C-mannosylation of tryptophan. J Biol Chem 2009;284:30004-15. [Crossref] [PubMed]
- Verbij FC, Stokhuijzen E, Kaijen PH, et al. Identification of glycans on plasma-derived ADAMTS13. Blood 2016;128:e51-8. [Crossref] [PubMed]
- Hofsteenge J, Huwiler KG, Macek B, et al. C-mannosylation and O-fucosylation of the thrombospondin type 1 module. J Biol Chem 2001;276:6485-98. [Crossref] [PubMed]
- Eura Y, Kokame K, Takafuta T, et al. Candidate gene analysis using genomic quantitative PCR: identification of ADAMTS13 large deletions in two patients with Upshaw-Schulman syndrome. Mol Genet Genomic Med 2014;2:240-4. [Crossref] [PubMed]
- Deforche L, Roose E, Vandenbulcke A, et al. Linker regions and flexibility around the metalloprotease domain account for conformational activation of ADAMTS-13. J Thromb Haemost 2015;13:2063-75. [Crossref] [PubMed]
- von Krogh AS, Kremer Hovinga JA, Tjønnfjord GE, et al. The impact of congenital thrombotic thrombocytopenic purpura on pregnancy complications. Thromb Haemost 2014;111:1180-3. [Crossref] [PubMed]
- Moatti-Cohen M, Garrec C, Wolf M, et al. Unexpected frequency of Upshaw-Schulman syndrome in pregnancy-onset thrombotic thrombocytopenic purpura. Blood 2012;119:5888-97. [Crossref] [PubMed]
- Sakai K, Fujimura Y, Miyata T, et al. Current prophylactic plasma infusion protocols do not adequately prevent long-term cumulative organ damage in the Japanese congenital thrombotic thrombocytopenic purpura cohort. Br J Haematol 2021;194:444-52. [Crossref] [PubMed]
- Yagi H, Konno M, Kinoshita S, et al. Plasma of patients with Upshaw-Schulman syndrome, a congenital deficiency of von Willebrand factor-cleaving protease activity, enhances the aggregation of normal platelets under high shear stress. Br J Haematol 2001;115:991-7. [Crossref] [PubMed]
- Furlan M, Robles R, Morselli B, et al. Recovery and half-life of von Willebrand factor-cleaving protease after plasma therapy in patients with thrombotic thrombocytopenic purpura. Thromb Haemost 1999;81:8-13. [Crossref] [PubMed]
- Taylor A, Vendramin C, Oosterholt S, et al. Pharmacokinetics of plasma infusion in congenital thrombotic thrombocytopenic purpura. J Thromb Haemost 2019;17:88-98. [Crossref] [PubMed]
- Zheng XL, Vesely SK, Cataland SR, et al. ISTH guidelines for treatment of thrombotic thrombocytopenic purpura. J Thromb Haemost 2020;18:2496-502. [Crossref] [PubMed]
- Kovarova P, Hrdlickova R, Blahutova S, et al. ADAMTS13 kinetics after therapeutic plasma exchange and plasma infusion in patients with Upshaw-Schulman syndrome. J Clin Apher 2019;34:13-20. [Crossref] [PubMed]
- Kinoshita S, Yoshioka A, Park YD, et al. Upshaw-Schulman syndrome revisited: a concept of congenital thrombotic thrombocytopenic purpura. Int J Hematol 2001;74:101-8. [Crossref] [PubMed]
- Naik S, Mahoney DH. Successful treatment of congenital TTP with a novel approach using plasma-derived factor VIII. J Pediatr Hematol Oncol 2013;35:551-3. [Crossref] [PubMed]
- Scully M, Gattens M, Khair K, et al. The use of intermediate purity factor VIII concentrate BPL 8Y as prophylaxis and treatment in congenital thrombotic thrombocytopenic purpura. Br J Haematol 2006;135:101-4. [Crossref] [PubMed]
- McGonigle AM, Patel EU, Waters KM, et al. Solvent detergent treated pooled plasma and reduction of allergic transfusion reactions. Transfusion 2020;60:54-61. [Crossref] [PubMed]
- Sidhu D, Snyder EL, Tormey CA. Two approaches to the clinical dilemma of treating TTP with therapeutic plasma exchange in patients with a history of anaphylactic reactions to plasma. J Clin Apher 2017;32:158-62. [Crossref] [PubMed]
- Garraud O, Malot S, Herbrecht R, et al. Amotosalen-inactivated fresh frozen plasma is comparable to solvent-detergent inactivated plasma to treat thrombotic thrombocytopenic purpura. Transfus Apher Sci 2019;58:102665. [Crossref] [PubMed]
- Raval JS, Padmanabhan A, Kremer Hovinga JA, et al. Development of a clinically significant ADAMTS13 inhibitor in a patient with hereditary thrombotic thrombocytopenic purpura. Am J Hematol 2015;90:E22. [Crossref] [PubMed]
- Fujimura Y, Lämmle B, Tanabe S, et al. Patent ductus arteriosus generates neonatal hemolytic jaundice with thrombocytopenia in Upshaw-Schulman syndrome. Blood Adv 2019;3:3191-5. [Crossref] [PubMed]
- Camilleri RS, Scully M, Thomas M, et al. A phenotype-genotype correlation of ADAMTS13 mutations in congenital thrombotic thrombocytopenic purpura patients treated in the United Kingdom. J Thromb Haemost 2012;10:1792-801. [Crossref] [PubMed]
- Fujimura Y, Matsumoto M, Kokame K, et al. Pregnancy-induced thrombocytopenia and TTP, and the risk of fetal death, in Upshaw-Schulman syndrome: a series of 15 pregnancies in 9 genotyped patients. Br J Haematol 2009;144:742-54. [Crossref] [PubMed]
- Sakai K, Fujimura Y, Nagata Y, et al. Success and limitations of plasma treatment in pregnant women with congenital thrombotic thrombocytopenic purpura. J Thromb Haemost 2020;18:2929-41. [Crossref] [PubMed]
- Miodownik S, Pikovsky O, Erez O, et al. Unfolding the pathophysiology of congenital thrombotic thrombocytopenic purpura in pregnancy: lessons from a cluster of familial cases. Am J Obstet Gynecol 2021;225:177.e1-177.e15. [Crossref] [PubMed]
- Scully M, Thomas M, Underwood M, et al. Thrombotic thrombocytopenic purpura and pregnancy: presentation, management, and subsequent pregnancy outcomes. Blood 2014;124:211-9. [Crossref] [PubMed]
- Yoshida Y, Matsumoto M, Yagi H, et al. Severe reduction of free-form ADAMTS13, unbound to von Willebrand factor, in plasma of patients with HELLP syndrome. Blood Adv 2017;1:1628-31. [Crossref] [PubMed]
- Sánchez-Luceros A, Meschengieser SS, Marchese C, et al. Factor VIII and von Willebrand factor changes during normal pregnancy and puerperium. Blood Coagul Fibrinolysis 2003;14:647-51. [Crossref] [PubMed]
- Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med 1991;325:393-7. [Crossref] [PubMed]
- Kayashima M, Sakai K, Harada K, et al. Strong association between insufficient plasma exchange and fatal outcomes in Japanese patients with immune-mediated thrombotic thrombocytopenic purpura. Int J Hematol 2021;114:415-23. [Crossref] [PubMed]
- Péquériaux NC, Fijnheer R, Gemen EF, et al. Plasma concentration of von Willebrand factor predicts mortality in patients on chronic renal replacement therapy. Nephrol Dial Transplant 2012;27:2452-7. [Crossref] [PubMed]
- Holden RM, Tuttle A, Burbidge T, et al. Quantitative and qualitative changes of von Willebrand factor and their impact on mortality in patients with end-stage kidney disease. Blood Coagul Fibrinolysis 2013;24:719-26. [Crossref] [PubMed]
- Ocak G, Roest M, Verhaar MC, et al. Von Willebrand factor, ADAMTS13 and mortality in dialysis patients. BMC Nephrol 2021;22:222. [Crossref] [PubMed]
- Green D, Roberts PR, New DI, et al. Sudden cardiac death in hemodialysis patients: an in-depth review. Am J Kidney Dis 2011;57:921-9. [Crossref] [PubMed]
- Makar MS, Pun PH. Sudden Cardiac Death Among Hemodialysis Patients. Am J Kidney Dis 2017;69:684-95. [Crossref] [PubMed]
- Itami H, Hara S, Matsumoto M, et al. Complement activation associated with ADAMTS13 deficiency may contribute to the characteristic glomerular manifestations in Upshaw-Schulman syndrome. Thromb Res 2018;170:148-55. [Crossref] [PubMed]
- Borogovac A, Tarasco E, Kremer Hovinga JA, et al. Prevalence of neuropsychiatric symptoms and stroke in patients with hereditary thrombotic thrombocytopenic purpura. Blood 2022;140:785-9. [Crossref] [PubMed]
- Upreti H, Kasmani J, Dane K, et al. Reduced ADAMTS13 activity during TTP remission is associated with stroke in TTP survivors. Blood 2019;134:1037-45. [Crossref] [PubMed]
- Tanabe S, Fujimura Y, Lämmle B, et al. Stealth thrombosis of brain and kidney in a girl with Upshaw-Schulman syndrome not receiving prophylactic plasma infusions. Int J Hematol 2020;112:603-4. [Crossref] [PubMed]
- Tarasco E, Bütikofer L, Friedman KD, et al. Annual incidence and severity of acute episodes in hereditary thrombotic thrombocytopenic purpura. Blood 2021;137:3563-75. [Crossref] [PubMed]
- Scully M, Knöbl P, Kentouche K, et al. Recombinant ADAMTS-13: first-in-human pharmacokinetics and safety in congenital thrombotic thrombocytopenic purpura. Blood 2017;130:2055-63. [Crossref] [PubMed]
- Schiviz A, Wuersch K, Piskernik C, et al. A new mouse model mimicking thrombotic thrombocytopenic purpura: correction of symptoms by recombinant human ADAMTS13. Blood 2012;119:6128-35. [Crossref] [PubMed]
- Nathwani AC, Reiss UM, Tuddenham EG, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014;371:1994-2004. [Crossref] [PubMed]
- Pasi KJ, Rangarajan S, Mitchell N, et al. Multiyear Follow-up of AAV5-hFVIII-SQ Gene Therapy for Hemophilia A. N Engl J Med 2020;382:29-40. [Crossref] [PubMed]
- Biffi A, Montini E, Lorioli L, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 2013;341:1233158. [Crossref] [PubMed]
- Hacein-Bey-Abina S, Pai SY, Gaspar HB, et al. A modified γ-retrovirus vector for X-linked severe combined immunodeficiency. N Engl J Med 2014;371:1407-17. [Crossref] [PubMed]
- Cook K, Forbes SP, Adamski K, et al. Assessing the potential cost-effectiveness of a gene therapy for the treatment of hemophilia A. J Med Econ 2020;23:501-12. [Crossref] [PubMed]
- Laje P, Shang D, Cao W, et al. Correction of murine ADAMTS13 deficiency by hematopoietic progenitor cell-mediated gene therapy. Blood 2009;113:2172-80. [Crossref] [PubMed]
- Niiya M, Endo M, Shang D, et al. Correction of ADAMTS13 deficiency by in utero gene transfer of lentiviral vector encoding ADAMTS13 genes. Mol Ther 2009;17:34-41. [Crossref] [PubMed]
- Trionfini P, Tomasoni S, Galbusera M, et al. Adenoviral-mediated gene transfer restores plasma ADAMTS13 antigen and activity in ADAMTS13 knockout mice. Gene Ther 2009;16:1373-9. [Crossref] [PubMed]
- Jin SY, Xiao J, Bao J, et al. AAV-mediated expression of an ADAMTS13 variant prevents shigatoxin-induced thrombotic thrombocytopenic purpura. Blood 2013;121:3825-9, S1-3.
- Verhenne S, Vandeputte N, Pareyn I, et al. Long-Term Prevention of Congenital Thrombotic Thrombocytopenic Purpura in ADAMTS13 Knockout Mice by Sleeping Beauty Transposon-Mediated Gene Therapy. Arterioscler Thromb Vasc Biol 2017;37:836-44. [Crossref] [PubMed]
- Leebeek FWG, Miesbach W. Gene therapy for hemophilia: a review on clinical benefit, limitations, and remaining issues. Blood 2021;138:923-31. [Crossref] [PubMed]
Cite this article as: Sakai K, Hamada E, Kokame K, Matsumoto M. Congenital thrombotic thrombocytopenic purpura: genetics and emerging therapies. Ann Blood 2023;8:24.


