Acquired hemophilia A and deep vein thrombosis attributable to the Pfizer-BioNTech SARS-CoV-2 mRNA vaccine—case report
Introduction
Acquired hemophilia A (AHA) is a rare autoimmune disorder that causes bleeding in patients with no family or personal history of bleeding and affects about 1.48 people per million per year (1). The natural history and management approach of AHA are distinct from those of the inherited form of hemophilia A. Hence, there is need for a high-index of suspicion in patients who develop unexplained bleeding and have conditions known to cause or predispose to AHA. Conditions such as autoimmune disease (including rheumatoid arthritis, systemic lupus erythematosus) and cancers are commonly associated with AHA (2,3). Other factors known to be causally related to AHA include pregnancy or the puerperium, and certain drug reactions (penicillin, sulfonamides, phenytoin) (3). Furthermore, older adults are reported to be at greater risk of developing AHA (3,4). However, the specific cause or trigger of AHA remains unidentified in almost 50% of cases (3,4). Due to the high case fatality rate of AHA (ranging from 7.9% to 22%) within the first few weeks of manifestation, prompt diagnosis and treatment are essential (4).
Over the last two years, coronavirus disease 2019 (COVID-19), which is caused by the SARS-CoV-2 virus, has emerged as a rapidly spreading infectious disease with primary respiratory pathology but associated with multisystemic manifestations (5). Its impact on the economic and healthcare infrastructure of every nation has been substantial and unprecedented. At the time of writing this report, over 270 million cases of COVID-19 have been recorded globally, with over 5 million COVID-19 deaths (6). The advent of effective vaccines against the SARS-CoV-2 virus was welcomed globally amidst concerns about their long-term safety. In the United States, the use of novel methods of vaccine development, particularly mRNA vaccine technology, was a key factor that led to the rapid development and deployment of the Pfizer-BioNTech and Moderna mRNA vaccines against SARS-CoV-2 (7), each of which has over 90% efficacy and a good safety profile (7,8). COVID-19 vaccines have been instrumental in slowing the rate of new COVID-19 infections and reducing disease severity in the United States and globally, and are the major tools aimed at controlling the COVID-19 pandemic (8). At least two doses of each of these mRNA vaccines are needed for substantial immunity.
In addition to the aforementioned causes of AHA, a few post-vaccination cases have been reported, particularly following influenza vaccination, while one case of AHA has been reported following COVID-19 vaccination with the Pfizer-BioNTech SARS-CoV-2 mRNA vaccine (9). In addition, although venous thromboembolism—occurring as either pulmonary embolism or as deep venous thrombosis (DVT)—is a frequently reported complication of COVID-19 infection, venous thromboembolism following COVID-19 vaccination is a rare occurrence, with only two cases of thromboembolic events so far reported following COVID-19 vaccination (10,11). In light of the need for adequate vaccination coverage to mitigate the burden of COVID-19 at the population level, as well as the need to provide high-value individualized care, awareness of rare adverse events of COVID-19 vaccination is necessary for early diagnosis and treatment of patients who develop those adverse events and to maintain the public’s trust in the safety and effectiveness of the vaccines. We, therefore, report a case of AHA and DVT following administration of the Pfizer-BioNTech SARS-CoV-2 mRNA vaccine in accordance with the CARE reporting checklist (available at https://aob.amegroups.com/article/view/10.21037/aob-21-66/rc).
Case presentation
A 63-year-old male with medical history of hypertension, former tobacco use and hyperlipidemia presented one week after receiving the first dose of the Pfizer-BioNTech SARS-CoV-2 mRNA vaccine with a 3-day history of left lower extremity (LLE) swelling and pain. On presentation, he was hemodynamically stable. Physical examination was significant for tenderness at the calf of the LLE on palpation and passive range of motion. He also had 2+ pitting edema up to the left knee, and ecchymosis to the distal mid left thigh and posterior calf. Complete blood count revealed hemoglobin concentration of 11.9 g/dL, platelet count of 242×109/L, while activated partial thromboplastin time (APTT) was elevated to 68.0 seconds. Venous Doppler ultrasound showed acute DVT in the left popliteal vein, while CT of the LLE showed evidence of acute intermuscular fascial edema which was thought to be due to extravasation from interruption in flow by clot. He did not have personal or family history of hypercoagulability, no recent surgery, prolonged travel, immobilization or history of malignancy. Work up for malignancy with CT of the chest, abdomen and pelvis was negative. Given no prior personal or family history or risk factors for provoked DVT, patient’s DVT was deemed unprovoked.
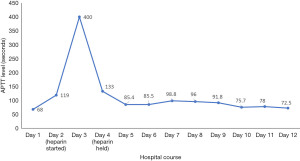
The patient was admitted and started on heparin drip based on an assessment of unprovoked DVT and clinical suspicion of spontaneous gastrocnemius hematoma, with plan to monitor for compartmental syndrome (versus phlegmasia). By day 3, the patient had a supratherapeutic APTT of >400 seconds, as well as punctate ecchymoses from blood draw sites and progression of ecchymoses of posterior calf and posterior thigh of the LLE. The patient developed hypotension, necessitating intravenous fluid boluses. By day 4, he had developed multiple hematomas in the left upper and lower extremities, and acute post-hemorrhagic anemia with hemoglobin concentration of 7.3 g/dL, necessitating transfusion of 1 unit of packed red blood cells (PRBC) that resulted in post-transfusion hemoglobin concentration of 8.8 g/dL. With further drop in hemoglobin, the decision was made to stop the heparin drip on day 4, as the initial lower extremity swelling was thought to be from a hematoma and the clot was likely due to stasis. A CT scan of the abdomen and pelvis did not show any hematoma. In addition, a repeat venous Doppler ultrasound of the bilateral lower extremities on day 5 was negative for DVT. An inferior vena cava (IVC) filter was subsequently placed on day 5. Despite discontinuing heparin, the patient continued to have markedly elevated APTT as shown in Figure 1.
The patient had increasing swelling and ecchymoses of the left arm and developed a spontaneous hematoma of the right forearm. By days 5 through 7, he had developed hematomas in all four extremities and his hemoglobin concentration dropped to 6.5 g/dL, necessitating another PRBC transfusion. With multiple sites of hematoma and persistent blood loss and elevated APTT with recent vaccination, consideration was for bleeding disorders, disseminated intravascular coagulopathy (DIC) and autoimmune etiology and the possibility of vaccine induced coagulation disorder. Retrospective history for personal of family history of bleeding and autoimmune disorders was negative. Workup for DIC was negative. Von Willebrand Factor (vWF), antiphospholipid antibodies, lupus anticoagulant, TSH, anti-ds DNA, and hepatitis serology testing were negative. Results of the patient’s mixing study are shown in Table 1, with partial correction of APTT. We further obtained factor VIII assay, as shown in Table 2, which was consistent with acquired factor VIII inhibitor (acquired hemophilia A).
Table 1
| Test | Value (seconds) | Reference range (seconds) |
|---|---|---|
| APTT initial patient | 103.2 | 24.4–33.4 |
| APTT pool plasma | 31.5 | – |
| Immediate APTT 1:1 mixing | 43.0 | <33.5 |
| Incubated APTT 1:1 mixing | 56.5 | <37.3 |
Mixing study showed partial correction of APTT immediately with gradual rise in 1 hour. APTT, activated partial thromboplastin time.
Table 2
| Factor VIII assays | Results |
|---|---|
| Factor VIII activity | <1 |
| Factor VIII inhibitor EIA | Positive |
| Factor VIII activity clotting | <1 |
| Nijimegen assay | 69.6 |
EIA, enzyme immunoassay.
Treatment with Factor Eight Inhibitor Bypassing Activity (FEIBA) at 100 units/kg every 12 hours was started immediately, and subsequently tapered and discontinued in three days upon resolution of active bleeding. To eliminate factor VIII inhibitor, immunosuppressive therapy was initiated with IV methylprednisolone 80 mg daily and cyclophosphamide 2 mg/kg daily. His hemoglobin concentration subsequently remained stable around 8.4–8.9 g/dL. On day 12, the patient felt much improvement in pain and swelling of extremities, and he was discharged home on oral prednisone and cyclophosphamide slow taper, with scheduled outpatient hematology follow-up in 1 week. Figure 2 summarizes the timeline from vaccine administration and development of symptoms to diagnosis and recovery. Repeat labs done in the outpatient office showed improved hemoglobin concentration to 12.5 g/dL, when his prednisone was tapered off, whereas cyclophosphamide was continued for a few more weeks.

All procedures performed in this case report were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Acquired hemophilia A is caused by autoantibodies against coagulation factor VIII that partially or completely limit its procoagulant function (12). AHA has been associated with other autoimmune conditions, underlying malignancy, infections, medications (4,13) and rarely vaccinations (14)—few cases have been attributed to seasonal influenza vaccines. The pathophysiology of post-vaccination AHA is unclear, although it has been suggested that vaccination can trigger an autoimmune response due to antigenic mimicry as well as due to activation of quiescent auto-reactive T and B cells (14), leading to production of autoantibodies (15). More than two-thirds of AHA cases present with severe bleeding. As seen in our patient, the management includes controlling the bleeding episode with bypassing agents such as recombinant activated factor VIII or activated prothrombin complex concentrate, anti-inhibitor complex (FEIBA) and eradication of the inhibitor with immunosuppressive therapy (16). It is also proposed that an intense immunological response evoked by the vaccine could be a trigger for the thrombotic event—this is the mechanism recognized in many clinical conditions (11).
The most common adverse effects reported with COVID-19 vaccination are pain at injection site, fever, chills, arthralgia, myalgia, headache, and anaphylaxis—the most serious of which is anaphylaxis (17). The SARS-CoV-2 virus is known to induce a hypercoagulable state, such that venous thromboembolism is a significant cause of morbidity and mortality in COVID-19 patients (18,19). So far, only one case of AHA and two cases of DVT have been reported following COVID-19 vaccination amongst more than 8 billion COVID-19 vaccines administered worldwide (6). In each case, the Pfizer-BioNTech SARS-CoV-2 mRNA vaccine was implicated. Hitherto, there has been no report of both DVT and AHA occurring in the same patient after COVID-19 vaccination, making this the first known report of the co-occurrence of such life-threatening adverse events associated with COVID-19 vaccination.
We acknowledge that it is almost impossible to establish a definitive link between the COVID-19 vaccine and the co-occurrence of AHA and DVT via a single case report; however, studies suggest strongly that vaccination can trigger autoimmune responses, therefore supporting this possibility.
Conclusions
The purpose of this case report is to raise awareness among healthcare workers about possible rare adverse events like DVT and AHA that can be associated with the Pfizer-BioNTech SARS-CoV-2 mRNA vaccine and the treatment of AHA with bypassing agents, like FEIBA, and immunosuppression with steroids and cyclophosphamide. Considering that vaccination is currently the major tool aimed at controlling the COVID-19 pandemic, it is imperative to report such rare, life-threatening adverse events that could occur shortly after receiving the vaccine. To reduce these events, it could be beneficial to assess for DVT and bleeding risks prior to administration of the Pfizer-BioNTech SARS-CoV-2 mRNA vaccine. Close follow-up of high-risk patients within the first few weeks following administration of the first vaccine dose will help ensure early diagnosis and treatment of AHA and/or DVT, should they occur.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://aob.amegroups.com/article/view/10.21037/aob-21-66/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-21-66/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this case report were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Collins PW, Hirsch S, Baglin TP, et al. Acquired hemophilia A in the United Kingdom: a 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors’ Organisation. Blood 2007;109:1870-7. [Crossref] [PubMed]
- Boggio LN, Green D. Acquired hemophilia. Rev Clin Exp Hematol 2001;5:389-404; quiz following 431.
- Green D, Lechner K. A survey of 215 non-hemophilic patients with inhibitors to Factor VIII. Thromb Haemost 1981;45:200-3.
- Franchini M, Gandini G, Di Paolantonio T, et al. Acquired hemophilia A: a concise review. Am J Hematol 2005;80:55-63. [Crossref] [PubMed]
- White-Dzuro G, Gibson LE, Zazzeron L, et al. Multisystem effects of COVID-19: a concise review for practitioners. Postgrad Med 2021;133:20-7. [Crossref] [PubMed]
- Johns Hopkins University of Medicine Coronavirus Resource Center. COVID-19 Dashboard. Johns Hopkins University website. Accessed December 19, 2021. Available online: https://coronavirus.jhu.edu/map.html
- Huang Q, Zeng J, Yan J. COVID-19 mRNA vaccines. J Genet Genomics 2021;48:107-14. [Crossref] [PubMed]
- Moghadas SM, Vilches TN, Zhang K, et al. The Impact of Vaccination on Coronavirus Disease 2019 (COVID-19) Outbreaks in the United States. Clin Infect Dis 2021;73:2257-64. [Crossref] [PubMed]
- Radwi M, Farsi S. A case report of acquired hemophilia following COVID-19 vaccine. J Thromb Haemost 2021;19:1515-8. [Crossref] [PubMed]
- Al-Maqbali JS, Al Rasbi S, Kashoub MS, et al. A 59-Year-Old Woman with Extensive Deep Vein Thrombosis and Pulmonary Thromboembolism 7 Days Following a First Dose of the Pfizer-BioNTech BNT162b2 mRNA COVID-19 Vaccine. Am J Case Rep 2021;22:e932946. [Crossref] [PubMed]
- Carli G, Nichele I, Ruggeri M, et al. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern Emerg Med 2021;16:803-4. [Crossref] [PubMed]
- Oh J, Lim Y, Jang MJ, et al. Characterization of anti-factor VIII antibody in a patient with acquired hemophilia A. Blood Res 2013;48:58-62. [Crossref] [PubMed]
- Franchini M, Glingani C, De Donno G, et al. The first case of acquired hemophilia A associated with SARS-CoV-2 infection. Am J Hematol 2020;95:E197-8. [Crossref] [PubMed]
- Wraith DC, Goldman M, Lambert PH. Vaccination and autoimmune disease: what is the evidence? Lancet 2003;362:1659-66. [Crossref] [PubMed]
- Mahendra A, Padiolleau-Lefevre S, Kaveri SV, et al. Do proteolytic antibodies complete the panoply of the autoimmune response in acquired haemophilia A? Br J Haematol 2012;156:3-12. [Crossref] [PubMed]
- Sallah S. Treatment of acquired haemophilia with factor eight inhibitor bypassing activity. Haemophilia 2004;10:169-73. [Crossref] [PubMed]
- Wadman M. Public needs to prep for vaccine side effects. Science 2020;370:1022. [Crossref] [PubMed]
- Schulman S, Hu Y, Konstantinides S. Venous Thromboembolism in COVID-19. Thromb Haemost 2020;120:1642-53. [Crossref] [PubMed]
- Porfidia A, Valeriani E, Pola R, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res 2020;196:67-74. [Crossref] [PubMed]
Cite this article as: Rani P, Ogunleye OO, Ramineni S, Medapati U, Berenzon DP. Acquired hemophilia A and deep vein thrombosis attributable to the Pfizer-BioNTech SARS-CoV-2 mRNA vaccine—case report. Ann Blood 2023;8:19.