
Lymphocyte response and recovery to radiation therapy alone
Introduction
Lymphocytes are considered one of the most radiosensitive of the mammalian cells. One reason for this is that they die an interphase death, meaning unlike most cells, death is immediate rather than requiring an attempt at cell division (mitotic death). As a result of this exquisite sensitivity, the lymphocyte count is considered an early measure of whole body radiation exposure and its severity, particularly with accidental exposure. Exact quantitation is difficult because the details of the exposure are often lacking, specifically, type of radiation and dose.
It has been long recognized that therapeutic radiation treatments can have a dramatic effect on lymphocyte counts. The consequences of radiation induced lymphopenia have been long debated, clear detrimental effects have not been demonstrated. For example, there is no clear risk of opportunistic infections as seen with other lymphocyte disorders such as human immunodeficiency virus (HIV) infection.
The question is now being readdressed due to the increasing interest in the role of lymphocytes in the prevention and treatment of cancer. There are concerns that radiation induced lymphopenia might be detrimental, but there are indications that other positive effects may also occur, such as a heightened antigenic state, or eradiation of immunosuppressive cells.
In spite of the millions of radiation therapy treatments given over the years, there is actually a paucity of clinical data on the effects of radiation treatment on lymphocyte counts. Most of the published data often includes radiation combined with chemotherapy. Since chemotherapy alone is known to be dramatically immunosuppressive, it is tenuous at best to make any firm conclusions on the effect of the radiation alone on the blood counts, so there is a data void.
Our goal was to capture the effect of radiation therapy on the lymphocyte counts without the confounding effect of chemotherapy. We report on over 300 prostate cancer patients treated with radiation therapy without chemotherapy. We present the following article in accordance with the STROBE reporting checklist (available at https://aob.amegroups.com/article/view/10.21037/aob-21-74/rc).
Methods
Historically, in treating the pelvis in patients with radiation alone, there was no reported risk on the blood counts and it was not matter of practice to routinely monitor the counts. With the adoption of intensity modulated radiation therapy (IMRT), while it allows for a higher total focused dose, it requires spreading out more dose through a much larger volume of normal tissue. This raises the question as to whether this larger irradiated volume increases the detriment to blood counts. Observationally, we had noticed that in some patients who had blood counts done, there was a significant drop in lymphocyte count. With this concern, in late 2014 we started routinely and uniformly collecting a baseline complete blood count (CBC) prior to treatment, at the end of treatment, and 3 months later in our prostate cancer patients. Empirically, by 2016, we had not seen any overly concerning trends and stopped the routine collection. We are retrospectively evaluating this prospective data to quantify the effect on lymphocytes and to determine if we could detect any dose/volume effects of the radiation therapy. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Baylor Scott and White Research (No. 021-136) and individual consent for this retrospective analysis was waived.
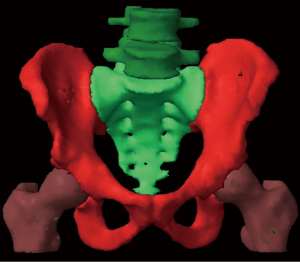
We could identify 301 prostate cancer patients in whom blood counts were obtained. These were a cross section of older patients receiving primary radiation and younger patients receiving post-operative radiation. A patient needed at least two of the count data points (baseline, end of treatment, 3 months) to be included. There were 291 baseline counts, 285 end of treatment counts and 282 three month counts. All patients were treated in a single institution. Patient characteristics are shown in Table 1. Patients consisted of those receiving primary prostate radiation therapy (n=193) or those receiving post radical prostatectomy radiation therapy (n=108). IMRT was delivered via linear accelerator. The majority (83%) received lymphatic (pelvic) radiation to a dose of 54 Gy (@ 1.8 Gy daily). The primary prostate patients usually received 78 Gy total dose, although some (n=22) received external beam therapy followed by an implant boost and a few rare patients in this time period underwent hypofractionated radiation therapy (n=4). One patient underwent hypofractionated boost. Post-operative patients received 70 Gy to the prostate fossa. Pelvic lymphatics were defined by the named vascular vessels: common iliac, external iliac, and internal iliac. Some patients received concomitant androgen ablation, but we previously determined (1) that this did not affect the radiation response of the blood components. In all the patients the pelvic bone was outlined from the inferior ischium to the top of L4 (“bone volume”). We also outlined the soft tissue for the same levels (total volume). The core components were L4-5, sacrum, bilateral os coxae and upper femurs (down to the level of the inferior ischium) (Figure 1). We determined the volume for each incremental dose (dose-volume), i.e., for 10 Gy, 20 Gy, 30 Gy, etc. (Figure 2 for the 20 Gy dose level volume) We then correlated this with the depletion of the lymphocyte count by the end of treatment and subsequent recovery. To try to gauge the severity of the lymphocyte suppression, we evaluated not only the absolute decline, but how that related to normal laboratory levels and common terminology criteria for adverse events 5.0 (CTCAE) (2). For lymphocytes, our lab normal range is 0.72–4.32×109/L. For CTCAE, Grade 1 is 0.8×109/L to lower limits of normal, but since our lower limit of normal is below that (0.72×109/L) , we can have no grade 1. Grade 2 is 0.5−0.8×109/L, Grade 3 is 0.2–0.5×109/L and Grade 4 is <0.2×109/L. Above 0.8×109/L is considered no toxicity, which we called Grade 0.
Table 1
| Variable | Value |
|---|---|
| Age (years) | |
| Mean | 69.3 |
| Median | 69.5 |
| Concomitant ADT | |
| Yes | 49.5% |
| No | 50.5% |
| Lymphatics treated (pelvis) | |
| Yes | 83% |
| No | 17% |
| Prostate/prostate fossa dose† | |
| 78 Gy | 55.2% |
| 70 Gy | 35.9% |
†, 27 patients received other (see text). ADT, androgen deprivation therapy.
Statistical analysis
We determined significant changes in lymphocytes over time using the Wilcoxon signed-rank test. Spearman correlations were used to examine the relationship between lymphocyte decline and dose volumes. To determine the dose volumes that maximize the sensitivity and specificity of grade 2 and 3 lymphocyte toxicity, we used logistic regressions and their resultant receiver operator characteristic (ROC) curves. Statistical significance was determined by P values less than 0.05. All statistical analyses were performed in SAS 9.4. All available data were used with less than 10% of patients with missing data at each time point. A sensitivity analysis was performed and no significant differences were detected between patients with complete and missing data.
Results
The overall volumes treated to each dose level are shown in Table 2. The overall bone volume was both a mean and median of 1,670 cm3. For total volume (inclusion of the soft tissue), mean volume was 20,645 cm3 and median volume 19,566 cm3.
Table 2
| Gy | Pelvic bone | Whole volume | |||
|---|---|---|---|---|---|
| Mean (%) | Median (%) | Mean (%) | Median (%) | ||
| V10 | 70 | 77.0 | 53.0 | 58.0 | |
| V20 | 54.9 | 61.0 | 31.7 | 34.0 | |
| V30 | 37.4 | 42.0 | 16.7 | 18.0 | |
| V40 | 21.5 | 24.0 | 9.8 | 11.0 | |
| V50 | 10.7 | 12.0 | 6.1 | 7.0 | |
| V60 | 1.5 | 1.0 | 1.5 | 1.0 | |
| V70 | 0 | 0 | 2.6 | 1.0 | |
The lymphocyte counts significantly (P<0.0001) decreased from baseline (median 1.92×109/L) by a median −1.22×109/L to 0.67×109/L (range, −6.28 to 0.02×109/L, P<0.0001) at the end of treatment (Table 3). The counts recovered some (+0.23×109/L, range, −0.71 to 2.82×109/L) but remained significantly below baseline at 3 months (P<0.0001) with an absolute median difference of −0.94×109/L (range, −3.54 to 0.27×109/L, P<0.0001). This represented a median change of −50.13% from baseline at 3 months. With longer follow up (mean 28 months) the lymphocytes recovered slightly, so that they were −0.77×109/L (−43%) below baseline levels. Only 62% had counts beyond 3 months and they were sporadic, although that subgroup was no different than the entire cohort as to dose, whether the pelvis was treated and their baseline, end of treatment and 3 months blood counts. Patients receiving primary radiation were a mean of 71.6 years of age and post-operative patients were 65.8 years. On linear regression analysis, age did not impact the lymphocyte count at any of the time measures. As a measurement of toxicity, at the start of treatment, 98% of the patients had lymphocyte counts in our laboratory’s normal range (0.72–4.32×109/L). For those within the normal range, 60% dropped below normal, with 28% below normal at 3 months. On the basis of common toxicity criteria, at baseline, 99% had no toxicity. At the end of treatment 32% (n=88) had no toxicity, 44% grade 2 (n=120), 23% grade 3 (n=63) and 1% grade 4 (n=30). By 3 months, there was significant recovery to where 65% (n=174) had no toxicity, 29% grade 2 (n=78) and 6% (n=17) grade 3, with no grade 4. With longer follow up, 78% were with no toxicity, 18% with grade 2 and 4% with grade 3. As another measure with the longer follow up, 86% of those that started in the normal range at baseline were in the normal range with 14% (n=40) below normal.
Table 3
| Grade | Cut point (%) | Sensitivity | Specificity | Positive predictive value |
Negative predictive value |
Accuracy | Risk below cut point (%) |
Risk above cut point (%) |
|
|---|---|---|---|---|---|---|---|---|---|
| Grade 2 | |||||||||
| V10 | 76 | 0.6443 | 0.6044 | 0.7764 | 0.4435 | 0.6316 | 56 | 78 | |
| V20 | 61 | 0.6340 | 0.6044 | 0.7736 | 0.4365 | 0.6246 | 56 | 77 | |
| V30 | 42 | 0.6134 | 0.6044 | 0.7677 | 0.4231 | 0.6105 | 58 | 77 | |
| V40 | 24 | 0.6289 | 0.6154 | 0.7771 | 0.4375 | 0.6246 | 56 | 78 | |
| V50 | 12 | 0.5876 | 0.6264 | 0.7703 | 0.4161 | 0.6000 | 58 | 77 | |
| Grade 3 | |||||||||
| V10 | 78 | 0.6377 | 0.6157 | 0.3465 | 0.8418 | 0.6211 | 16 | 35 | |
| V20 | 63 | 0.6087 | 0.6157 | 0.3360 | 0.8313 | 0.6140 | 17 | 34 | |
| V30 | 43 | 0.6377 | 0.5926 | 0.3333 | 0.8366 | 0.6035 | 16 | 33 | |
| V40 | 25 | 0.6232 | 0.6065 | 0.3359 | 0.8344 | 0.6105 | 17 | 34 | |
| V50 | 13 | 0.5362 | 0.5972 | 0.2984 | 0.8012 | 0.5825 | 20 | 30 | |
We next ascertained the effect of the dose and volume on counts. For both the pelvic bone volume and the whole volume with the soft tissue, at each increasing volume at every dose (V10–V50) there was a negative effect on counts (P<0.0001), based on spearman correlations. There was no correlation with V60 and V70 as the volumes that got those doses was very small (on the average only 1.5% of the bone received 60 Gy and less than 1% received 70 Gy). On linear regression analysis, at each dose level, each incremental increase in volume resulted in a greater decrease in lymphocytes. To determine the level of maximal detriment, logistical regression analysis for Grade 2 and 3 toxicity was performed for each dose level maximizing sensitivity (true positive) and specificity (true negative) rates. Results for each dose/volume to the bone are shown in Table 3 (for whole volume see Table S1).
Recognizing that the biggest difference in treated volumes was between those with lymphatic (n=250) and prostate/prostate fossa only (n=51) radiation, we evaluated the differences on lymphocyte counts (Table 4). The patients with the larger fields had a deeper drop that was more persistent. For the prostate/prostate fossa only patients the decline at 3 months was −35% (median) versus −54% for the pelvis treated patients. Only 24% of the prostate/prostate fossa treated patients developed grade 2 CTCAE toxicity (no grade 3 or 4), while 48% of the pelvis treated developed grade 2 (n=109), 28% grade 3 (n=63) and 1% (n=3) grade 4 toxicity. At 3 months, for prostate/prostate fossa only patients, 95% had no toxicity, 2% (n=1) with grade 2 and 2% (n=1) with grade 3 versus 59% with no toxicity, 34% (n=77) with grade 2 and 7% (n=16) with grade 3 in the large volume patients. Based on our normal levels, for those starting in the normal range, for the prostate/prostate fossa only patients, 11% were below normal the end of treatment, but 2% at 3 months. For the pelvis patients 69% dropped below normal, with 33% at 3 months.
Table 4
| Time | Prostate/prostate fossa only, ×109/L | Includes whole pelvis, ×109/L | All patients, ×109/L | Change interval | |||||
|---|---|---|---|---|---|---|---|---|---|
| Median | Change | Median | Change | Median | Change | ||||
| Baseline | 2.04 | −0.86 | 1.92 | −1.25 | 1.92 | −1.22 | Baseline to end | ||
| End of treatment | 1.03 | +0.2 | 0.62 | +0.23 | 0.67 | +0.23 | End to 3 months | ||
| 3 months | 1.36 | −0.73 | 0.85 | −0.99 | 0.93 | −0.94 | Baseline to 3 months | ||
Discussion
Lymphocytes are extremely sensitive to radiation, to the point that almost any amount of radiation will cause a decrease in numbers. Much of the cited data comes from laboratory cell culture and animal studies with the always questionable application to clinical human experience. The two best human sources are from the whole body radiation experience, either pre bone marrow transplant or accidental whole body radiation. With accidental whole body exposure, the International Atomic Energy Agency (IAEA) has calculated (3) that for single doses (considering a normal lymphocyte count to be approximately 2.0×109/L), that 1–2 Gy will result in a lymphocyte count of 0.7–1.5×109/L at 3–6 days, with doses of 2–4 Gy resulting in counts down to 0.5–0.8×109/L, 4–6 Gy to 0.3–0.5×109/L, 6–8 Gy to 0.1–0.3×109/L and >8 Gy to 0–0.1×109/L. Clearly, doses much above 8 Gy in a single exposure would be considered ablative. This has been supported by the bone marrow transplant experience. With whole body radiation prior to transplant, the goal is to totally eradicate all host immune cells, this would include not only the mature cells, but also precursor (i.e., stem) cells. A mitigating factor in most of the data is that these patients usually have leukemia, so the lymphocytes are not normal. Additionally, these patients have usually received preconditioning chemotherapy. Therefore, the radiation doses to achieve a similar cellular eradication would likely be somewhat higher. Ultimately, for fractionated radiation, it appears 15 Gy over 3 days is the most effective in complete eradication [86% successful in one study (4)], but to lessen toxicity, 12 Gy is usually used. With 15 Gy, there are still some failures, so a totally ablative dose with fractionated radiation would be higher than this. As with accidental exposure, it has been found that the drop in lymphocyte numbers is rapid. With just a single dose of 1.35–1.4 Gy, the lymphocyte count drops to 65% of pretreatment levels, most of that within the first 4 hours (5). In another study with hematologic malignancies (6), due to the preconditioning chemotherapy the lymphocyte count had already decreased from 2.2×109/L to 1.06×109/L. Then, after a single dose of 2 Gy the counts dropped to 0.67×109/L (further 38% reduction) and after a second 2 Gy treatment to 0.5×109/L (52% reduction). With the consideration that there was already a 50% lymphocyte reduction from the chemotherapy, this further 50% reduction is similar to the total reduction at the end of our small volume (prostate/prostate fossa) treatment. If we consider 15 Gy to be ablative for lymphocytes, the reduction of lymphocytes in our patients to 35% of baseline would indicate that 65% of the lymphocyte population had received at least a cumulative dose of 15 Gy or greater during the course of treatment.
With whole body radiation, the entire lymphocyte volume is irradiated. It is a much more complex with partial body radiation. For lymphocytes, the target volumes are the blood volume, the lymphatics, and the bone marrow [with consideration that the bone/bone marrow contains about 5% of the blood volume (7)]. Therefore, radiation to increasing volumes of these structures will collectively have a greater effect on lymphocyte numbers. At any given time, the pelvis contains about 15% of the total blood (7) volume. Also, the pelvis contains about 52% of the active bone marrow with 13% (6.6–10.1%) in the lumbar spine, 9% (7.5–9.9%) in the sacrum, 22% (15.5–25.3%) in the os coxae and 8% (4.5–12.8%) in the proximal femurs (7-9). The bone marrow itself is static, but the blood flow through it and in the rest of the pelvis (as with lymphatic flow) is not. The exact volume of the lymphatics/lymph fluid is uncertain, but anatomically, the major lymphatic vessels are considered to parallel the vascular system (10,11). Blood recirculates system wide every 30–60 seconds. For large arteries, like the iliac arteries, flow is very rapid, estimated at 20–50 cm2. With the typical “beam on” time for whole pelvis treatment of about 3 minutes, multiple volumes of blood get radiated daily. The daily exposure for blood in small vessels, such as capillaries, with a blood flow of 0.05–0.1 cm/s will be much higher (7). Lymph flow is mostly passive, but more than the entire lymph fluid volume is considered to re-enter and exit the vascular volume each day (12). Obviously, the larger the volume of each of these treated reservoirs will dictate the daily exposure. Field size does correlate with level of lymphopenia, even in the lung where not a lot of active marrow is treated (13). Ultimately, total dose to the lymphocytes are then determined by total number of fractions. The number of fractions is likely more important than the daily or even total dose given the extreme radiosensitivity of lymphocytes. Indeed, it appears that stereotactic body radiation therapy (SBRT), for example in the case of lung cancer, with 3–5 fractions and comparably smaller field sizes, results in a markedly less decrease in lymphocyte counts compared to more protracted, larger field radiation (14). There is still a significant drop with the smaller fields. In another SBRT study (15), the lymphocyte count dropped 24%, which improved to only 16% below baseline at 3 months.
With our larger treatment fields, overall, we confirmed a significant decline in lymphocyte count with a median decline from baseline of 50% by the end of treatment (Table 3). The 3-month recovery was modest and the counts were still below baseline at 3 months in 43% of patients. While interesting, it remains to be seen whether this is clinically relevant. We tried to ascertain this is two ways—first whether it took patients from being normal to abnormal based on their absolute counts and second, whether these changes would be considered “toxicity”. Based on normal lymphocyte levels from our lab, for those that were in the normal range to begin with, 60% dropped below normal, but by 3 months, about half of those returned to normal (leaving 28% below normal). Using common terminology toxicity criteria, at the end of treatment, 44% suffered grade 2 toxicity with 23% and 1% with the more serious grade 3 and 4 toxicity, respectively. By three months, this improved to where there was no grade 4 toxicity with 29% grade 2 and 6% grade 3. It is unclear what this “toxicity” really means clinically.
These findings were without the negative influence of chemotherapy. Similarly, most of the comparative literature is in the treatment in prostate cancer as most other cancers are treated with chemotherapy either before or with radiation therapy, if radiation therapy is given at all.
There are a few other contemporary studies. In a study (16) with 101 patients with large volume (>100 cm2) radiation without chemotherapy to diffuse sites, the lymphocytes dropped 50%. Interesting, the counts dropped 33% in week one and then plateaued by week 3. In patients (n=10) treated for seminoma (17) with a modest 26 Gy para-aortic radiation the baseline lymphocyte counts dropped from 1.4×109 to 0.65×109 by the midpoint (−54%) and 0.46×109 (−67%) at the end. Noted that the counts had improved to 0.86×109 at 4 months (37 % below baseline). In a small study (18) (10 primary and 23 post-operative) of prostate cancer patients, they reported that the base line lymphocyte levels were lower than healthy controls. For primary prostate patients, the lymphocyte counts dropped from 1.7 to 1.0×109/L (−42%) and from post-operative patients from 1.5 to 0.9×109/L (−40%) At a year, the counts had risen to 1.4×109/L and 1.2×109/L, respectively. Finally, in a study (19) of 121 prostate cancer patients with pelvic radiation and boost to the prostate/prostate fossa, lymphocyte count at the end was 30% of baseline. In general, it appears that with radiation alone utilizing standard doses and treatment, the lymphocyte drops by 40–50%, although the last study reports −70%.
We attempted to correlate the actual volume doses to outcome. Given the overlap in volume for each of the doses (for example all of the volume treated to 50 Gy was also treated with 40, 30, 20 and 10 Gy), this was an imprecise process. Based on the sensitivity and specificity (hence the accuracy) of the drop in counts, we determined “cut points” for grade 2 and 3 toxicity (Table 4). None was significantly better than the others and the overlap was significant. Still, if one wanted to put constraints on the volumes, these would be reasonable numbers to use (Table 4). It became clear that the differences were due to the different volumes treated for the whole pelvis with prostate boost patients, versus those treated to the prostate/prostate fossa only (“large volume” versus “small volume” patients, respectively).
In our series, the small volume patients had only a modest amount of bone (bone marrow) and lymphatic volume covered, so the acute effects are mostly driven primarily by radiation to the blood volume. The effects were still significant, but the effects were more pronounced when the larger volumes were treated (Table 3), with about a 45% greater decline (−1.25×109/L) for larger volumes, versus −0.86×109/L for the smaller. Interestingly, the absolute recovery at 3 months was about the same (+0.2×109 /L). This would suggest a fixed regenerative cell response in spite of the differing volumes treated. Again, we looked at whether these differences had any clinical relevance. For the small volume patients, 11% had below normal counts at the end of treatment with 2% at 3 months. For CTCAE metrics, there was no grade 3 or 4 toxicity and 24% had grade 2. At 3 months, there was only 1 patient (2%) each with grade 2 and grade 3 toxicity. With the large volume treatment, 69% had counts drop below normal and 33% were still below normal at 3 months. Forty eight percent developed grade 2, 28% grade 3 and 1% grade 4 toxicity. This improved, but at 3 months there was 34% with grade 2 and 7% with grade 3 toxicity.
As noted above, in studies where weekly counts are obtained, that the lymphocyte counts drop rapidly, but reach a nadir and relative steady state by the fourth week. Given the sensitivity of lymphocytes and that the entire blood volume was repeatedly treated, this new homeostasis is likely is a reflection of the redistribution from non-vascular locations into the blood pool and the steady state regenerative rate. It is remarkable that after two months of daily radiation (sans weekends) that the lymphocyte pool is not exhausted. With this, it is interesting to consider the experience with physically depleting lymphocytes prior to transplant (20). The lymphocyte concentration in the lymph fluid was about 10 time higher than the peripheral blood (30.0×109 /L), which dropped to a steady state of approximately 4×109/L over 3–6 days. With that, in the 14 patients’ vascular lymphocyte level also dropped, from approximately 2.1×109/L to a steady state of 0.70×109/L. This was at the expense of the central pool of lymphocytes. For example, in calves (n=48) (21), subjected to extra corporeal radiation for up to 50 hours, after 15 hours, vascular lymphocyte counts were down to 28%, and thoracic duct lymph fluid lymphocytes down to 38%. Again, there was a rapid decline and then a relative steady state. Histological analysis showed lymph tissue had a rapid decline in resident lymphocytes, some areas more than others. This experience further supports our observation that there is an initial rapid decline and then a relative steady state. In the above cited human clinical studies, there is then a slow replenishment. In our experience, by 3 months, there was some recovery, but less than 25% of what was lost. As we followed patients a little longer (mean 2 years), there is some gradual further improvement. There is not much long-term data, but some older studies suggest that a small number of patients have permanent detriment (22,23). There are almost no data with modern radiation techniques. The implications of delayed or incomplete lymphocyte recovery are uncertain. With the large number of patients treated and the almost universal effect of resultant lymphocyte reduction, the lack of reports on an increase in systemic opportunistic infections would indicate it is not clinically significant. It may be, as with our patients, the decline is rarely below normal levels for very long, and that the remaining counts are adequate. The disadvantage in our study is that we do not have longer-term follow up, so cannot comment on ultimate lymphocyte recovery.
Without chemotherapy, clinical radiation therapy results in a uniform and rapid decline in vascular lymphocytes, to a steady state 30–50% of baseline, with an initial rapid, but slower complete recovery. Larger fields of treatment result in a deeper decline, but similar recovery rate. The vast majority of patients recover with lymphocytes in the normal range without any obvious toxicity.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://aob.amegroups.com/article/view/10.21037/aob-21-74/rc
Data Sharing Statement: Available at https://aob.amegroups.com/article/view/10.21037/aob-21-74/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-21-74/coif). The authors have no conflicts of interest declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swanson G, Hammonds K, Jhavar S. Short-Term Haematogical Effects of Androgen Deprivation and Radiotherapy in Prostate Cancer Patients. Open Journal of Urology 2021;1:103-11.
- Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_8.5x11.pdf (Accessed on June 25, 2021).
- International Atomic Energy Agency, Diagnosis and Treatment of Radiation Injuries, Safety Reports Series No. 2, IAEA, Vienna (1998).
- Clift RA, Buckner CD, Appelbaum FR, et al. Long-term follow-Up of a randomized trial of two irradiation regimens for patients receiving allogeneic marrow transplants during first remission of acute myeloid leukemia. Blood 1998;92:1455-6.
- Girinsky T, Socie G, Cosset JM, et al. Blood lymphocyte subsets after the first fraction in patients given hyperfractionated total body irradiation for bone marrow transplantation. Br J Cancer 1991;63:646-7. [Crossref] [PubMed]
- Clave E, Socié G, Cosset JM, et al. Multicolor flow cytometry analysis of blood cell subsets in patients given total body irradiation before bone marrow transplantation. Int J Radiat Oncol Biol Phys 1995;33:881-6. [Crossref] [PubMed]
- Basic anatomical and physiological data for use in radiological protection: reference values. A report of age- and gender-related differences in the anatomical and physiological characteristics of reference individuals. ICRP Publication 89. Ann ICRP 2002;32:5-265.
- Hayman JA, Callahan JW, Herschtal A, et al. Distribution of proliferating bone marrow in adult cancer patients determined using FLT-PET imaging. Int J Radiat Oncol Biol Phys 2011;79:847-52. [Crossref] [PubMed]
- Brindle JM, Trindade AA, Shah AP, et al. Linear regression model for predicting patient-specific total skeletal spongiosa volume for use in molecular radiotherapy dosimetry. J Nucl Med 2006;47:1875-83.
- Margaris KN, Black RA. Modelling the lymphatic system: challenges and opportunities. J R Soc Interface 2012;9:601-12. [Crossref] [PubMed]
- Swanson GP, Hubbard JK. A better understanding of lymphatic drainage of the prostate with modern imaging and surgical techniques. Clin Genitourin Cancer 2013;11:431-40. [Crossref] [PubMed]
- Westermann J, Pabst R. Distribution of lymphocyte subsets and natural killer cells in the human body. Clin Investig 1992;70:539-44. [Crossref] [PubMed]
- Tang C, Liao Z, Gomez D, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys 2014;89:1084-91. [Crossref] [PubMed]
- Wild AT, Herman JM, Dholakia AS, et al. Lymphocyte-Sparing Effect of Stereotactic Body Radiation Therapy in Patients With Unresectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys 2016;94:571-9. [Crossref] [PubMed]
- Rutkowski J, Ślebioda T, Kmieć Z, et al. Changes in systemic immune response after stereotactic ablative radiotherapy. Preliminary results of a prospective study in patients with early lung cancer. Pol Arch Intern Med 2017;127:245-53. [Crossref] [PubMed]
- Lundgren M, Salete F, Cavalcanti M, et al. Weekly monitoring of the effects of conventional external beam radiation therapy on patients with head and neck, chest, and pelvis cancer by means of blood cells count. Radiologia Brasileira 2008;41:29-33.
- Belka C, Ottinger H, Kreuzfelder E, et al. Impact of localized radiotherapy on blood immune cells counts and function in humans. Radiother Oncol 1999;50:199-204. [Crossref] [PubMed]
- Sage EK, Schmid TE, Geinitz H, et al. Effects of definitive and salvage radiotherapy on the distribution of lymphocyte subpopulations in prostate cancer patients. Strahlenther Onkol 2017;193:648-55. [Crossref] [PubMed]
- Sini C, Fiorino C, Perna L, et al. Dose-volume effects for pelvic bone marrow in predicting hematological toxicity in prostate cancer radiotherapy with pelvic node irradiation. Radiother Oncol 2016;118:79-84. [Crossref] [PubMed]
- Tilney NL, Murray JE. Chronic thoracic duct fistula: operative technic and physiologic effects in man. Ann Surg 1968;167:1-8. [Crossref] [PubMed]
- Ruchti C, Cottier H, Cronkite EP, et al. Studies on lymphocytes. XVII. Differential lymphocyte depletion in lymphoreticular organs of the calf during continuous extracorporeal x-irradiation of the circulating blood. Cell Tissue Kinet 1970;3:301-15.
- Fuks Z, Strober S, Bobrove AM, et al. Long term effects of radiation of T and B lymphocytes in peripheral blood of patients with Hodgkin's disease. J Clin Invest 1976;58:803-14. [Crossref] [PubMed]
- Toivanen A, Granberg I, Nordman E. Lymphocyte subpopulations in patients with breast cancer after postoperative radiotherapy. Cancer 1984;54:2919-23. [Crossref] [PubMed]
Cite this article as: Swanson GP, Hammonds K, Jhavar S. Lymphocyte response and recovery to radiation therapy alone. Ann Blood 2023;8:2.


