
Patient blood management—it is about transfusing blood appropriately
Introduction
One of the earliest descriptions of patient blood management (PBM) is found in the medical bylaws of Providence Hospital in 1953. The medical staff adopted the following “indications for blood transfusion”: (I) to replace needed whole blood volume; (II) for oxygen transport: (i) with an anemic patient, otherwise well must have ≤7 g/dL hemoglobin (Hb), (ii) with an anemic patient with complications affecting oxygenation or is to undergo anesthesia may be transfused if the Hb is <10 g/dL; (III) for exchange transfusion; and (IV) if fresh whole blood is indicated in rare cases of dyscrasias where a labile element is important. The authors then stated that between 1953 and 1960, the application of these indications had resulted in a decreasing “use factor” (units transfused annually divided by number of patients) from 0.237 to 0.110, or by more than 50%. The stated benefits were the following: limiting transfusions to patients with valid medical indications, protecting patients from hazards of unnecessary transfusions, and easing the burden of donor recruitment and blood collection (1). This was very forward thinking in an era when the only relevant transfusion transmitted infection (RTTI) testing performed on blood donations was for syphilis.
In 1980—27 years later—the term “transfusion trigger” (2) was coined in an effort to examine the physician’s decision to transfuse blood. Early efforts to curb unnecessary transfusion practices were spurred by the recognition of adverse events related to the newly identified RTTIs associated with allogeneic blood. In the 1980s and 1990s, providers were taught the old adage, “if you are going to give one, you might as well give two”, stemming from a desire to avoid infectious risks of human immunodeficiency virus and hepatitis during the 1980s, when it was largely believed that single-unit transfusions were unnecessary. This policy was intended to decrease unneeded transfusions, but likely resulted in increased blood utilization. Rather than eliminating superfluous transfusions (which could be avoided by using restrictive transfusion thresholds), it encouraged over-transfusion in patients who would have benefited sufficiently from a single unit (3).
More recently, concerns about blood components have included transfusion-related immunomodulation risks and the red blood cell (RBC) storage lesion [current studies have not addressed RBC older than standard issue—days 35–42]. The more fundamental question regarding the efficacy of RBC and plasma transfusions remains (4).
Starting a PBM program can be a daunting task that requires support from hospital administration, information technology (IT) teams, medical and nursing staff, and the transfusion service. A structured and organized approach is necessary to include and coordinate necessary components in an effective manner. Most programs start with a charter that describes key stakeholders, primary goals, and metrics to evaluate success. One article listed nine key steps for implementing a PBM program (Table 1) (5). One of the first steps is to assemble a multidisciplinary team of clinicians and stakeholders. While most PBM and transfusion committees include the traditional blood users as well as laboratory and transfusion service personnel, it is also critical to include a representative from IT. As IT is often inundated by requests from physicians for reports, this will assist with an understanding that PBM is an important, evidence-based field where their efforts will be used in a meaningful way to change and improve medical practice within the hospital. IT’s early involvement in a PBM program is extremely important for its eventual success. Until the last decade it was very difficult to extract a large amount of transfusion data as from medical records, as most were on paper. This made the process arduous and feasible for only auditing a random sample of transfusion guideline for compliance. With the initiation of electronic medical records (EMRs) at most institutions, large amounts of transfusion data are available, yet extraction remains a challenge (6).
Table 1
| Obtain support from health system leadership with a business plan |
| Assemble multidisciplinary team of clinicians and stakeholders |
| Education (with emphasis on the randomized clinical trials supporting restrictive transfusion) |
| Harmonize transfusion guidelines for hospital or health system |
| Clinical decision support for computerized clinician order entry (with best practice advisories) |
| Data acquisition and analytics |
| Create guideline compliance dashboards |
| Transfusion guideline compliance audits with feedback (reports) to clinicians |
| Methods for improving blood use |
| ● Evidence-based transfusion thresholds |
| ● “Why give 2 when 1 will do” Choosing Wisely campaign for RBC transfusions |
| ● Preoperative anemia management for elective surgery (e.g., oral or IV iron, or erythropoietin) |
| ● Antifibrinolytics to reduce blood loss (e.g., aminocaproic acid or TXA) |
| ● Intraoperative autologous transfusion (cell salvage) |
| ● Anesthetic management (e.g., autologous normovolemic hemodilution, controlled hypotension, and normothermia) |
| ● Surgical methods (e.g., newer cautery methods, topical hemostatics, and sealants) |
| ● Reduce phlebotomy blood loss (e.g., use microtainers and reduce unnecessary laboratory tests) |
| ● Point of care testing (e.g., thromboelastography) |
PBM, patient blood management; RBC, red blood cell; IV, intravenous; TXA, tranexamic acid.
While some EMR systems provide their own reporting tools on blood utilization, a hospital may have multiple computer systems and databases (e.g., inpatient, outpatient, laboratory, blood bank, etc.) making the reporting tool for one EMR incomplete. It should be noted that not all EMRs are equal. Some software systems may be tailored to PBM at the time of purchase while other systems will require the hospital IT team to map data and develop specific reports from scratch. Often when hospitals are focused on getting a new EMR implemented for the entire hospital, they do not always anticipate the need to capture PBM data and therefore do not include the ability to do so during implementation. This creates a headwind for the implementation of a PBM program and often results in an overworked IT team developing a patchwork of reports that may not properly support the needs of a particular PBM program (7).
Once PBM informatics are in place, it is important to use the data to educate clinicians about transfusion guidelines and clinical evidence from randomized controlled trials (RCTs) supporting restrictive transfusion. A robust program for PBM education program can create buy-in for adherence to the guidelines (5,6). Peer-to-peer comparison of rates of compliance with the most current guidelines is an effective method for encouraging quality improvement and reducing inappropriate transfusions after clinicians have been educated (7).
Once clinicians are educated, it is important to monitor adherence to hospital transfusion guidelines through compliance audits with feedback (reports) to clinicians. Each clinical service should select a champion or leader who will receive and review a monthly report of inpatient transfusion orders. The champion or leader is responsible for providing feedback to colleagues. In addition, the report should be provided to hospital leadership, who will also have the opportunity to discuss the results with the service champions and to observe rates and trends of compliance with transfusion guidelines (5,6). Finally, other methods for improving appropriate blood utilization such as monitoring compliance with other PBM best practices, such as the “why give 2 when 1 will do” Choosing Wisely campaign for single unit RBC transfusions have been found to be beneficial (6).
Other important initiatives for PBM programs include: preoperative anemia management for elective surgery [e.g., oral or intravenous (IV) iron, or erythropoietin], antifibrinolytics to reduce blood loss [e.g., aminocaproic acid or tranexamic acid (TXA)], intraoperative autologous transfusion (cell salvage), anesthetic management (e.g., autologous normovolemic hemodilution, controlled hypotension, and normothermia), surgical methods (e.g., newer cautery methods, topical hemostatics, and sealants), the reduction of phlebotomy blood loss (e.g., use of microtainers and reduction of unnecessary laboratory tests), and point-of-care testing (e.g., viscoelastic hemostatic assays) (5).
In addition to the patient-related risks, PBM programs must consider the nation’s blood supply. The availability of safe blood is a key component of current medical practice (8). Although utilization is decreasing, more than 16% of Medicare claims include blood use (9). A robust, readily accessible blood supply is essential to support the United States (US) healthcare system and provide assurance that enough blood is available to meet daily patient needs (10). Currently, the US blood supply is almost entirely dependent on volunteer donors and a loose network of approximately 50 federally regulated non-profit blood centers, in addition to numerous hospital-based collection centers (10). In addition, the US Department of Defense (DoD) manages the Armed Services Blood Program that meets the needs of the military. This system has proved extraordinarily robust for more than 50 years (8). This loose network of blood centers has served the US well in the past, ensuring the safety and availability of blood needed every day for more than 4,000 hospitals. However, the continued availability of a robust blood supply faces significant threats and challenges in the current environment (10). A decade of decreased blood utilization and changes in health care delivery (e.g., new effective treatments for anemia, more endoscopic surgery, etc.) have ironically altered market conditions sufficiently to place the sustainability of the current system at risk and therefore making PBM increasingly more important and relevant (8).
Progress in appropriate blood utilization is being made. The percentage of US hospitalizations requiring RBC transfusions decreased from 6.8% in 2011 to 5.7% in 2014 (adjusted relative risk of 0.83). The percentage of US hospitalizations requiring plasma transfusions decreased from 1.0% in 2011 to 0.87% in 2014 (adjusted relative risk of 0.87) (8). The authors believed the decreases in RBC and plasma utilization from 2011 to 2014 may reflect evidence demonstrating the efficacy of restrictive practice for RBC transfusions, PBM programs, conservation initiatives (e.g., cell salvage, pharmacotherapy, improved surgical techniques), advocacy from medical organizations, and the publication of transfusion guidelines (11).
In conclusion, although transfusions can be a lifesaving therapy in patients who are hemorrhaging or severely anemic, unnecessary transfusions expose patients to increased risk and cost. Given that most of the evidence supporting a restrictive transfusion strategy has been published in the past decade, PBM programs have only recently gained popularity (5). A survey by the Association for the Advancement of Blood and Biotherapies (AABB) of practices in 2013 reported that only 38% of hospitals had a formal PBM program, highlighting the potential for growing PBM nationally to promote patient safety, improve quality of health care, and reduce unnecessary expenditure (5,12). Adherence to transfusion guidelines should be an institutional priority at every medical center. Widespread compliance with guidelines will result in increased quality as well as cost savings for patients, payers, and medical centers, as well as preservation of the blood supply for patients who truly need transfusions (5).
Transfusion review process—transfusion options
Deciding whether or not to transfuse allogeneic blood
One of the primary objectives of PBM is to minimize unnecessary blood transfusions, especially before planned surgeries. It can be achieved by adhering to appropriate transfusion thresholds and exploring relatively safer alternatives, before proceeding with allogeneic blood transfusion. Even though transfusion practices and quality standards have significantly improved with time, the risk of associated adverse events is always present. The implementation of an EMR system and educational feedback to the physician, were pivotal in decreasing inappropriate RBC transfusions in the US (13). There are automated programs available, called Clinical Decision Support Software (CDSS), which can be integrated into the physician order entry system and provide suggestions whenever an inappropriate blood order is detected. Implementing programs like CDSS has been shown to improve the transfusion practices in various studies and metanalysis (14) and can lay a robust foundation for establishing PBM at an institutional level.
Detection and management of pre-operative anemia plays an important role in minimizing the requirement for allogeneic transfusion. Even a mild degree of preoperative anemia has been independently associated with an increased risk of morbidity and mortality in patients undergoing major surgery (15). The European Society of Anesthesiology recommends that Hb levels should be measured 4 to 8 weeks before elective surgery, especially in patients who are at an increased risk of bleeding (16). Early detection provides sufficient time for treatment of anemia before the patient can be taken for elective surgery. A multi-disciplinary approach towards identifying such patients and timely management plays a crucial role in achieving this aim.
The 2018 PBM International Consensus Conference (ICC) (17) recommendations include iron supplementation in adult pre-operative patients with iron-deficiency anemia, as it is effective in improving Hb levels and reduces intraoperative RBC transfusion. Oral iron supplementation is the preferred mode of administration and IV administration may be required if the compliance is poor, absorption through oral route is impaired or time is limited (18). A Cochrane review published in 2019, compared six RCTs and concluded that iron supplementation alone did not reduce the risk of blood transfusion (19), but the smaller sample size included in the RCTs was cited as a major limitation of the review.
The use of short acting erythropoietin may be considered in certain clinical settings such as major orthopedic surgery with preoperative hemoglobin levels less than 13 g/dL but long acting erythropoiesis stimulating agents (ESAs) have not been recommended for routine use due to potential life-threatening side effects such as thromboembolic deep vein thrombosis (17). In addition to correction of pre-operative anemia, other measures to reduce the use of allogeneic blood include autologous transfusion. Preoperative autologous donation (PAD) may be offered to those patients who are in relatively good health, and expected to have a significant blood loss (>500 mL) during an upcoming planned surgery. As there are regulatory and financial implications associated with PAD (at least 50% or more of units collected are discarded), it is infrequently used.
Transfusion thresholds
Evidence based RBC transfusion thresholds in different pre-defined clinical settings is an integral part of a successful PBM program. Most of the available literature is based on observational or retrospective data and restricted only to specific clinical scenarios. AABB clinical practice guidelines published in 2016 (20), ICC Frankfurt [2018] (17) recommendations and a National Institute for Care and Excellence (21) guidelines are some of the recent important papers and all suggest a restrictive transfusion threshold of 7 g/dL for hemodynamically stable adult patients, including hospitalized critically ill patients. A restrictive RBC transfusion threshold of 8 g/dL is recommended for patients undergoing orthopedic surgery, cardiac surgery, and those with pre-existing cardiovascular disease. These threshold levels were found to be safe in most clinical settings and encouraged to be continued for future use as well. The transfusion thresholds for the pediatric population are dependent on different parameters including age and oxygen saturation, but a restrictive approach has been shown to have a similar survival rate and better safety profile in various studies (22).
Perioperative assessment of the surgical patient
Perioperative bleeding and blood transfusion is associated with poor survival outcome in various surgical settings. A retrospective analysis performed on American College of Surgeons National Surgical Quality Improvement Project (ACS-NSQIP) database, observed that “patients who were at the least risk for operative mortality or serious operative morbidity had the greatest odds of suffering an adverse surgical outcome if transfusions are given” (23).
Different surgical specialties have developed scoring systems that help in making objective and rational decisions when required. The Association of Cardiothoracic Anesthetists (ACTA) perioperative risk of blood transfusion score (ACTA-PORT score), is one such scoring system (24). A scoring system based approach towards blood transfusion helps in promoting rational use of blood products and should be encouraged in future as well.
Intraoperative techniques to reduce allogeneic transfusion
Intraoperative techniques to minimize blood loss may be broadly categorized into surgical methods, pharmacological intervention, cell salvage strategies, and acute normovolemic hemodilution (ANH). Minimally invasive surgical methods (laparoscopic and robotic surgeries) usually have lesser blood loss compared to open or more invasive approaches. Local bleeding may be controlled with the use of topical hemostatic agents including fibrin/thrombin gel.
Cell salvage comprises of collection, processing and re-administration of the autologous bloodshed during surgery. It can be performed during the intraoperative period or even in the postoperative period if needed. The Association of Anesthetists recommends using cell salvage when it can reduce the possibility of allogeneic red cell transfusion (25). Special precautions and case to case consideration must be exercised in surgeries involving infected wounds and cancer patients.
TXA has proven to be highly effective in controlling bleeding and minimizing transfusion requirement in different clinical settings, while use of other agents such as aprotinin and epsilon aminocaproic acid (EACA) are other options although used less frequently (26). Many RCTs and prospective studies have repeatedly concluded that TXA safely reduces the risk of death in bleeding patients and minimizes allogeneic blood transfusions.
In ANH, blood is collected by phlebotomy from the patient, at the start of surgery and the amount of blood removed is compensated with IV fluid (colloid or crystalloid) in order to maintain the isovolemic state. As the surgery nears completion, the phlebotomized blood is transfused back to the patient. ANH has been found effective in reducing allogeneic blood transfusion but can be used only in those scenarios where removal of blood before surgery is safe and feasible.
Postoperative strategies to minimize allogeneic transfusion
The blood collected through drains after surgery may be utilized for re-infusion once it has been processed. Postoperative blood salvage has been utilized in a wide variety of surgical procedures, but it is predominantly used after cardiac and orthopedic surgeries. The unwashed cell salvage may be associated with more risk factors which can be mitigated by using washed cell salvage and use of filters. Washed cell salvage has been recommended in patients who bleed more than 100 mL/h within the first 6 hours after cardiac surgery, and whenever re-sternotomy is required for hemorrhage (25). Other broad measures like minimizing iatrogenic blood loss during sample collection, point of care monitoring, prompt management of coagulopathy and maintaining adequate oxygen saturation levels help in avoiding unnecessary post-operative blood transfusion as well.
Massive transfusion and blood utilization
The current Massive Blood Transfusion (MTP) guidelines advocate transfusion of plasma, platelets, and RBCs in a ratio of 1:1:1, as per recommendations of the Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial (27). The recommendations from this trial have added to the findings of a previous study; the Prospective, Observational, Multicenter, Major Trauma Transfusion (PROMMTT), which concluded that higher plasma and platelet ratios early in resuscitation were associated with decreased mortality (28). As MTP is often initiated in an emergency, when to end the MTP depends mostly on clinical judgment and the termination point may not be well defined. There is always a risk of over-transfusion. Even in trauma centers with established MTP protocols, the incidence of over-transfusion may be as high as 27% (29). It will be prudent to consider the principles of PBM while implementing an Institutional MTP.
Blood utilization
Blood transfusion is a common hospital procedure, with nearly 10.9 million RBC units transfused in the US in 2019 (30). Although blood transfusion plays a critical role in patient care, overuse or inappropriate use of blood products may be associated with adverse patient outcomes and increased costs. Additionally, limiting unnecessary transfusions is an important way to be an effective steward of the blood supply and ensure that blood products will always be available for the patients that need them most.
Development and implementation of blood utilization guidelines and corresponding review processes is an effective way to identify potential areas for improvement in transfusion practice and patient safety within the organization.
The transfusion review process
Blood utilization review programs provide peer review of organizational transfusion or blood utilization practices and are an important component of a PBM program. The purpose of a utilization review program is to perform peer-review and evaluate adherence to institutional transfusion guidelines as well as to provide feedback that can be used for continuous quality improvement. In addition to reviewing transfusion ordering and administration practices, programs may also evaluate product expiration and wastage, patient safety and adverse events, and potential areas for cost reduction. Effective blood utilization review programs may be associated with lower transfusion risks, reduced costs, and improved quality outcomes (31).
Blood utilization review may be performed by the Transfusion Committee, the PBM Committee, or by an institutional quality or laboratory utilization committee. The committee that performs the review should include representation from the major departments that transfuse blood, which may include medicine, surgery, emergency medicine, pediatrics, and anesthesiology. Additionally, the transfusion service medical director, transfusion safety officer (TSO), the blood bank supervisor or a blood bank medical technologist, and representatives from hospital administration, nursing, quality assurance, IT, and the institution’s blood supplier may also be included in the committee (32). The committee should meet at least quarterly and reports from the committee should be made available to the medical staff (33).
Development of facility-specific transfusion practice guidelines
The first step in implementing a blood utilization review program is to establish a set of institution-specific, evidence-based transfusion practice guidelines for each blood component, including component modifications such as irradiation where appropriate. Additionally, separate guidelines may be developed for specific patient populations such as neonates and pediatrics, patients requiring massive transfusion, cardiothoracic surgery patients, or patients with sickle cell disease (33). The guidelines should be reviewed regularly and updated as new evidence becomes available. Evidence may include literature, recommendations from consensus conferences, practice statements from specialty societies, or evidence of standard practices at comparable institutions. While the guidelines provide an outline of best transfusion practice, they should also remain flexible to allow for certain patient circumstances which may warrant transfusion outside of the guidelines. The transfusion practice guidelines should be approved by the committee and shared with the medical staff.
Monitoring of physician ordering and transfusion effectiveness
Once local transfusion practice guidelines have been established and communicated to the medical staff, the blood utilization review program should establish audit criteria for monitoring of transfusion ordering practices. Audits should seek to identify outliers and repeated patterns of use that may require more careful investigation. In some circumstances, such as when the total number of events is small or when reports can be automated, it may be appropriate to review all transfusions while in other cases it may be appropriate to review only a representative sample of transfusions. In addition to overuse, audits should also be designed to evaluate for potential situations of undertransfusion.
Evaluation of processes involved in the preparation, dispensing, and administration of blood components
In addition to monitoring of transfusion ordering practices, the committee should also review and establish procedures related to the adequacy of the blood supply, pretransfusion testing, and administration of blood components (31). Audits should focus on patient safety, including areas that are known potential sources of error or areas in need of improvement. This may include:
- Blood product availability: including processes for managing during times of inventory constraint;
- Turn-around times: from sample collection to blood administration, including emergency transfusion situations;
- Specimen collection and labelling procedures: to ensure accuracy and reduce the risk of ABO incompatible transfusions due to wrong blood in tube (WBIT) errors;
- Informed patient consent: includes patient identification prior to administering a transfusion;
- Transfusion outcomes: including transfusion reactions and other adverse patient events;
- Appropriate use and maintenance of equipment: including blood warmers, cell salvage devices, and satellite blood refrigerators.
Data collection and quality improvement
Audit data should be analyzed and reported regularly. Sources of data may include patient medical records, laboratory information systems, or reports supplied from blood suppliers. Automation of data collection allows for evaluation of a larger amount of data and more complete analysis with less effort and cost than manual chart reviews. Peer review of the data is used to determine if deviations from organizational transfusion guidelines are clinically justified and may assist with identification of patterns of overutilization or underutilization that may necessitate intervention. If certain deviations are routinely justified, the committee may consider whether the guidelines should be modified to align with the acceptable institutional practice more closely.
When a potential area for improvement is identified, the committee should use the principles of continuous quality improvement to identify an appropriate intervention and evaluate the success of that intervention. If a physician or specific department is regularly ordering transfusions outside of approved guidelines, the committee may decide to meet with the individual or department representatives to provide education on the guidelines and why they are important. If turn-around times for blood delivery are too long and resulting in patient care delays or safety events, the committee may decide to map the process and identify steps that may be causing delay so that appropriate interventions can be implemented. After an intervention is implemented, the committee should continue to monitor performance metrics for a defined period to determine if the intervention was successful and, if so, that the progress is maintained.
Auditing blood utilization
Benchmarking, data analytics, metrics, utilization, medical economics, algorithms, measuring, converting raw data, big data, processed data, informatics—how can all these pieces of information be utilized in PBM to an end point that will change practice, promote patient safety and quality along with improving patient-focused healthcare? PBM includes several concepts that can and should be analyzed to make the right transfusion decisions for patients with the idea that avoidance, conservation and treatment of the underlying condition (i.e., anemia) should be the optimal way to individualize treatment (6).
Benchmarking is a continuous process by which an organization can measure and compare blood usage from the beginning of starting a focused PBM program. There are various kinds of benchmarking, including internal, competitive and functional. The function in PBM is transfusion of blood product or not transfusing, and the types of measurements will include productivity, quality, outcomes and costs related to the administration of blood products. Part of the startup activities of a PBM program, should be to acquire the utilization of blood products by service line and then by surgeon or physician. Since the development of EMRs in the last decade, extraction of data has become less daunting if IT departments are engaged early, with a well-thought out plan (6).
Conducting reviews of the data collection is the next step. Types of reviews include prospective, concurrent, retrospective, peer reviews, and a new concept—dashboards. Prospective reviews include the initial review to be conducted prior to the start of a treatment. One type of prospective review is the development of a Maximum Surgical Blood Order Set (MSBOS), which is a data driven protocol for determining which surgical patients need preoperative orders for blood. Many institutions use a MSBOS that may be based on consensus opinions rather than based on data for specific surgical procedures. An algorithm based on electronic data would include three variables—percentage of patients transfused, median estimated blood loss and average number of units transfused per patient—as Johns Hopkins describes for creating an institution-specific MSBOS (34). A dashboard which shows blood product inventory by product and type could also be considered a prospective review for pre-surgical blood usage. This method of review takes much coordination and communication between the transfusion service and the operating rooms that are performing high blood use surgery, but it can be used as a tool to prepare teams to be informed and utilize different strategies depending on the availability of blood products. The transfusion service can utilize this type of dashboard to maintain adequate blood product supply, modify orders to prevent shortages and excesses of inventory and transfer products between facilities in a system, either to prevent wastage of short-dated products or prevent cancelation of surgeries (35).
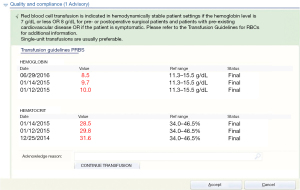
Concurrent review of transfusion services is conducted in “real time” during a patient’s course of treatment. This type of review could include the implementation of transfusion guidelines, especially in the EMR. Enthusiasm exists for practice guideline development and implementation, and some have even recommended that guidelines be used to certify and license physicians, but in PBM the implementation and review of adherence to the guidelines is important in measuring compliance (36). Simple interventions, which can include education, reminders, best practice alerts (BPAs) built into the EMR for each product, have been effective in changing physicians transfusion practices and reducing blood utilization (37). When a BPA appears for any blood product with the guideline stated and the clinical evidence (Figure 1) it is will change how a physician orders blood for a patient at the bedside (38). Electronic health record (EHR) blood ordering BPA can be used to challenge requests for blood products that do not meet the current guideline or “transfusion trigger” and give the provider the opportunity to review or rethink the order for the product. This type of alert to the provider has been shown to reduce inappropriate blood use as well as recording the decision to override the recommendation provided by the alert, which can initiate retrospective reviews of “overrides” (37).
Retrospective reviews, simply put, are conducted after the service to the patient has been rendered. Although being the easiest approach, this type of review has the disadvantage of losing the opportunity for effective intervention with the ordering physician. Retrospective review can be effective though, in analyzing trends in specific service lines to change practice. Hospitals that subscribe and submit statistics to a database can generate retrospective reports that can show the individualized blood product usage over time. When certain blood product use is increasing, then discussions can take place about treating anemia pre-operatively or addressing use of Direct Oral Anticoagulants (DOAC) prescribing pre-operatively. This type of analysis and implementation is patient-focused PBM.
Key performance indicators (KPIs) for blood transfusions that should be considered by the hospital transfusion committees include:
- % of patients transfused for the top 5–10 surgical procedures;
- % of patients transfused above the Hb threshold or hospital guideline;
- % of patients transfused to a Hb of 10 g/dL;
- % of double-unit transfusions (39).
This type of retrospective review can be used to compare one hospital with other similar hospitals using leading health care performance improvement organizations. Comparison of similar institutions or hospital systems can be helpful to clarify understanding of transfusion practices and the outcomes of certain surgeries as well as length of stay, readmission, infection rates and costs between hospitals that may utilize more blood products vs. those that use less blood products. PBM, with a focus on quality, safety and patient outcomes, should be considered when reviewing transfusion practices whether reviewing service-line outcomes or individual provider outcomes.
With the implementation of enhanced recovery after surgery (ERAS) throughout various countries, the optimization of patients prior to surgery includes collection of and analyzing laboratory values to identify undiagnosed diabetes, iron deficiency anemia and also coagulation concerns that may impact a patient’s ability to recover well from surgery. Preoperative anesthesia testing (PAT) clinics should include complete blood count (CBC), A1c, total iron binding capacity (TIBC), ferritin, B12, folate, international normalized ratio (INR), activated partial thromboplastin time (aPTT), and type and screen tests that will inform the clinicians overseeing the perioperative anemia clinic what conditions may need to be corrected for each patient prior to surgery, so that blood component transfusions can be avoided. Four of the 10 recommendations at the 2018 Frankfurt Consensus Conference on PBM include: (I) detection and management of preoperative anemia; (II) use of iron supplementation to reduce RBC transfusion; (III) do not use ESAs routinely in general for adult preoperative patients with anemia undergoing elective surgery; and (IV) consider short-acting erythropoietins in addition to iron supplementation to reduce transfusion rates in adult preoperative patients with hemoglobin concentrations <13 g/dL undergoing elective major orthopedic surgery (17). Correcting iron deficiency anemia, along with addressing possible coagulation concerns with patients on anticoagulants or antiplatelet medications should be a quality and safety initiative in the PAT clinics. Outcomes of optimized patients vs. those who do not get a preoperative assessment and intervention can be easily seen in increased length of stay, readmission rates, increased morbidity and mortality. A hospital’s data analytics department can easily extrapolate this type of data, along with the reduction in blood product usage.
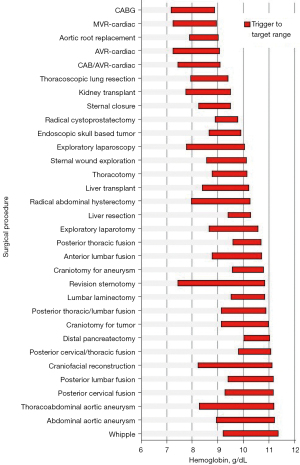
Extracting data on blood use in the operating room and effectively presenting that in a meaningful and effective way, possibly in the Transfusion Committee meetings or directly in peer review within surgeon’s own specialties, will have the greatest influence on changing practice (32). This was demonstrated by comparing mean transfusion hemoglobin thresholds and then targets for all surgeons and anesthesiologists who had >10 patients in a database. It was determined that presenting data in this manner was better received than when surgeons are compared by blood utilization such as percentage of patients transfused or average number of units transfused per patient (Figure 2) (6).
One area that is often overlooked when it comes to PBM initiatives, is hospital acquired anemia (HAA) from too much sampling. For every 50 mL of blood drawn, the Hb drops by 0.5 g/dL and the risk of moderate to severe HAA increases by 18%. A simple study in the intensive care unit (ICU) setting collecting data on blood sampling and including wastage from arterial lines can really be an eye opener to intensivists caring for these patients. Implementation of a closed blood sampling device or changing to smaller volume phlebotomy tubes can be a simple solution to causing anemia in already critically ill patients (40). Additionally, it is important to have the ability to compare a population of patients who refuse blood transfusions to those who receive blood products. This type of review can be done with the Society for Thoracic Surgery (STS) database and in specific cardiovascular procedures, such as isolated-coronary artery bypass grafts (CABG) (Figure 3). To implement successful PBM programs, institutions need to use the latest technology available to obtain and review the data required to improve practice and provide evidence-based outcomes to encourage best practice and reduce unnecessary transfusions.
Transfusion audit criteria
RBCs
RBCs are transfused to increase oxygen-carrying capacity in patients with anemia in whom physiologic compensatory mechanisms are inadequate to maintain normal tissue oxygenation (41). Worldwide there is a substantial variability in the transfusion thresholds of RBC. It is often the norm to base the decision to transfuse on the Hb levels of patients (42) due to limitation in methods to measure tissue oxygenation, although certain guidelines advocate the consideration of symptoms of anemia and not solely the Hb levels (43,44).
As the world moves towards “restrictive” transfusion practices which have not shown any evidence of harm to the patient when compared to a more liberal transfusion thresholds in various clinical trials (45-69), Clinical Practice Guidelines from the AABB recommend a Hb threshold of 7 g/dL for hospitalized adult patients who are hemodynamically stable, including critically ill patients, but a hemoglobin transfusion threshold of 8 g/dL for patients undergoing orthopedic or cardiac surgery and for those with underlying cardiovascular disease (20). It is emphasized that other variables such intravascular volume status, shortness of breath, exercise tolerance, light-headedness, chest pain thought to be cardiac in origin, hypotension, or tachycardia unresponsive to fluid challenge, and patient preferences should be taken into consideration before decision making rather than solely basing the decision to transfuse on Hb levels (20).
To audit the compliance for a standardized trigger for initiating transfusion and to offset the subjective variations in the blood orders based on physicians’ preference, it is always beneficial to decide the transfusion threshold of the facility and incorporate it in the computerized provider order entry (CPOE) with the capacity for CDSS. It is also pertinent for the facility to have a MSBOS in place so that appropriate usage of surgical transfusions can be ensured. Using such criteria for audit, the facility may also consider excluding from its review the RBC transfusions occurring in patients of thalassemia and sickle cell anemia who are transfused as outpatients. Currently there is still a paucity of evidence and clinical trials in chronically transfused patients, including in those with hematological malignancies and bone marrow failure syndromes, despite their high volume of blood use. In such patients, it is unclear whether current practices such as restrictive transfusion strategies are optimal (70).
The single unit transfusions have now been encouraged under the AABB’s Choosing Wisely campaign and Johns Hopkins Health Systems campaign “why give 2 when 1 will do?” (71,72). The audit should also capture data of undertransfusion as robust and aggressive PBM programs may lead to avoidance of transfusion by the provider.
Platelets
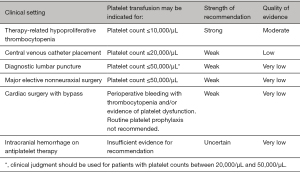
Platelet transfusions are indicated either as prophylactic in cases of hypoproliferative thrombocytopenia and before an invasive procedure, or therapeutic in cases of active bleeding. The threshold for prophylactic transfusion in hypoproliferative thrombocytopenia has been established to be 10,000/μL (73,74) on the basis of both observational studies (75,76) and randomized clinical trials (77-79). The recommended threshold for platelet transfusions for major invasive procedures is 40,000/μL to 50,000/μL and 20,000/μL for less invasive procedures such as central venous catheter insertion or removal and marrow aspiration or biopsies (80).
Platelet Transfusions are also indicated in thrombocytopenic patients with platelet count below 50,000/μL or at a higher count in patients with a qualitative platelet defect (41), however, the decision to transfuse should be guided by clinical judgment rather than platelet count alone.
The lower dose of platelets administered during prophylaxis has been shown as a safe alternative to standard dose of platelets but leads to an increase in the total number of transfusions given (81).
Failure to get the expected platelet increment post transfusion needs to be thoroughly investigated for the immune and non-immune causes using the parameters of corrected count increment and platelet count increment. HLA matched platelets may be needed in case the refractoriness is due to HLA antibodies. For a more efficient audit, the audit criteria of a transfusing facility may focus on transfusions of platelets done outside the accepted and implemented criteria. AABB has made recommendations for prophylactic platelet transfusions in adults (Figure 4) (38,41,73).
Plasma
Fresh frozen plasma (FFP) and other varieties like plasma frozen within 24 hours after phlebotomy (PF24), plasma cryoprecipitate reduced, and solvent/detergent-treated plasma (SD plasma) are primarily used for treating bleeding disorders arising out of single or multiple coagulation factor deficiencies, massive transfusion or for reversal of the vitamin K antagonists such as warfarin as also mentioned in evidence based guidelines issued by AABB. Despite these guidelines, there is lack of unequivocal conclusive evidence-based practice for plasma transfusions and hence there are widely divergent practices. The indications and effect of plasma can be gauged by the coagulation tests that include the prothrombin time (PT)/INR or aPTT in addition to clinical judgment. The dosage required for effective reversal of vitamin K antagonists is 15–20 mL/kg (41).
For a more efficient audit, the audit criteria on which a transfusing facility may focus on (82,83):
- Plasma transfusion in the context of massive transfusion;
- INR less than 2.0 in a nonbleeding patient scheduled for or undergoing surgery or an invasive procedure;
- Emergency reversal of warfarin anticoagulation in a patient with intracranial hemorrhage;
- Deficiency of specific factors of the coagulation system when appropriate factor concentrates are available.
The audit criteria may also include evaluation of the dose given and assessment whether the abnormality (such as elevated INR) was corrected. In addition, the audit may note the presence of heparin, hirudin analogs, or other drugs that may interfere with the coagulation system and/or its in-vitro assessment, a situation in which plasma transfusion will be ineffective.
Cryoprecipitated antihemophilic factor
The name cryoprecipitated antihemophilic factor, reflects the historical use of this product to stop the bleeding in patients of hemophilia A. With the advent of individual factor concentrates its use has become limited to certain acquired fibrinogen deficiencies (84) such as massive blood loss from trauma, hemorrhagic obstetric complications, and disseminated intravascular coagulation (DIC) (85) and when fibrinogen levels are found to be less than 100 mg/dL (86). In the setting of clinically significant bleeding after liver transplant cryoprecipitate transfusion is advised with a target fibrinogen level of 150 to 200 mg/dL (87). Cryoprecipitate obtained from one donor contains a minimum of 80 international units of factor VIII and 150 mg of fibrinogen and its transfusion dose of one unit per 10 kg body weight raises the plasma fibrinogen concentration by nearly 50 mg/dL if there is no associated consumption or massive bleeding (88). For a more efficient audit, the audit criteria of a transfusing facility may focus on transfusions of cryoprecipitate that occur when the fibrinogen level is more than 100 mg/dL.
Granulocytes
Prolonged severe neutropenia with intensive chemotherapy for hematologic malignancies or in the setting of hematopoietic stem cell therapy (HSCT) (defined as an absolute neutrophil count of <500/μL) predisposes patients to life-threatening bacterial and fungal infections despite aggressive antimicrobial therapy (89). Granulocyte transfusions have been hypothesized to be beneficial in such cases. Modern granulocyte transfusions aim to achieve the dosage of 6–8×1010 neutrophils per transfusion with lower limit of 4×1010 (90). Of note, as per AABB Standards for Blood Banks and Transfusion Services, a minimal dose of 1×1010 in at least 75% of units is required (91). The product should be ABO matched, Rh D compatible, cytomegalovirus (CMV) compatible, and irradiated and HLA matched in alloimmunized patients. It should be transfused as soon as possible and within 24 hours of collection without the use of leucocyte reduction filter (90).
The indications for granulocyte transfusions are not defined due to inconclusive evidence. Astute clinical judgment should augment the decision-making process for the transfusion trigger. The audit criteria should be developed in the transfusing facility should encompass reported indications, timelines of use, and donor stimulation strategies (33).
Criteria for specially modified blood components
Arguably, transfusion of blood and blood components are safer now than they have ever been. From advances in blood component preparation and storage, to advances in RTTI testing, the number of adverse events and fatalities from transfusions have declined significantly. Although the risk of adverse events and death have declined, the risk is still there and certainly not zero.
Infections and disease transmittance caused by pathogens in blood and blood components remains an issue in 21st century transfusion medicine. These events have been mitigated with donor screening questions, more advanced RTTI testing, and more recently pathogen reduction technology (92). With the frequency of TTD decreasing, the frequency of reporting of noninfectious complications has increased.
Leukocyte-reduced components
Noninfectious complications such as febrile nonhemolytic transfusion reactions, transfusion-associated graft-vs.-host disease (TA-GvHD), transfusion related acute lung injury (TRALI), HLA alloimmunization, platelet refractoriness, can be attributed to residual donor white blood cells. In addition to these noninfectious complications, some infectious viruses are spread only through leukocytes (93). These viruses include CMV and human T-cell lymphotropic virus I and II.
The vast majority of modern day transfusion practices utilize blood components rather than whole blood. The reason behind this is that not every patient needs everything in whole blood, such as RBCs, plasma, white blood cells, and platelets. Blood can be collected and separated into components, or apheresis devices can collect only the desired component and return the rest to the donor. Even with advances in cellular collection, there remains the possibility of contaminants in the collection. For example, platelet collections contain platelets and plasma, but also can contain a considerable about of white blood cells.
Leukocyte reduction of blood products involve filtering the leukocytes pre-storage. Leukocyte reduction filters remove leukocytes using two different techniques. In the first, the 4 μm filter permits the passing of RBC and platelets while trapping white blood cells. The negatively charged leukocytes also adhere to the filters through electrostatic and van der Waals forces. Due to the adhesion of leukocytes, larger pore sizes can be used for a higher flow rate through the filter. With the second method, the filters can be modified to create a larger positive charge, thus increasing the filter efficacy (94). On average, a unit of RBC can contain about 2 billion white blood cells. This is significantly reduced with leukoreduction. In order to be considered leukoreduced, a unit of RBCs must contain no more than 5×106 white blood cells (91,94,95).
Recent studies have shown that prestorage leukocyte reduction has also decreased the number of microvesicles in RBC units. Erythrocyte derived microvesicles are released as a normal part of aging, and due to cold storage of blood (96). These microvesicles are bioactive molecules that can activate the immune system and cause inflammatory effects. It has been shown that these microvesicles play a role in lung injury post resuscitation (96). Prestorage leukocyte reduction of RBC products reduced the number or erythrocyte derived microvesicles when compared to RBCs that were not leukocyte reduced.
CMV-reduced-risk cellular components
Components that are leukoreduced are considered CMV reduced risk or CMV safe components. Lymphotropic viruses, such as CMV, can only be spread through white blood cells. Significantly reducing the number of leukocytes in the product renders it CMV safe.
The literature suggests that leukocyte reduction and CMV seronegative blood products have similar but not identical efficacy, with an estimated transmission risk by seronegative components of 1% to 2% vs. a risk of 2% to 3% with leukocyte-reduced components (97-99). The major risk of transmission occurs in low-birthweight infants as a result of breastfeeding from a CMV-infected mother (99,100).
Oncology patients and neonatal patients are the main populations who require CMV negative products due to their compromised immune system. CMV negative products are considered safe for both populations and adequately mitigate the risk of infection or reinfection with a different strain.
Josephson and colleagues published a study in 2014 addressing CMV transmission in blood components in a neonatal population. Their study included very low birth weight neonate receiving both CMV seronegative and leukoreduced blood products. Neither group was associated with transmission of CMV from the blood products to the neonate. However, there was a correlation between CMV transmission from the mother’s breast milk to the neonate (100).
Irradiated blood components
One of the most damaging events a cell can undergo is a DNA double strand break. These events lead to apoptosis and or mutations within the cell. Irradiation of cellular components utilizes double strand breaks to inactivate white blood cells in the unit, preventing TA-GvHD. Please note that irradiated products are not equivalent to CMV safe.
TA-GvHD occurs when donor lymphocytes engraft in host tissues, and then further proliferate and mount an immune response against the recipient tissues. In healthy individuals, these lymphocytes are usually destroyed by the host’s immune system. In immunocompromised patients, or patients with similar HLA types as the donor, they are unable to destroy the donor white blood cells (101). Signs and symptoms of TA-GvHD include erythematous maculopapular rash, fever, pancytopenia, and hepatomegaly. Unfortunately, this condition usually results in fatality, making its prevention a high priority in transfusion medicine.
Irradiation of cellular components is done by emitting 25 Gy or equivalent to the center of the container, with a minimum dose of 15 Gy at any point of the container (91,102). This dose ensures enough damage has been done to the residual leukocytes to where they will not be able to replicate or cause further harm. Irradiation can be done to any component where a considerable number of white cells are present, i.e., RBC units, platelets, liquid plasma, and granulocyte apheresis.
One of the complications of irradiating blood is the effect it has on the storage lesion. RBCs that undergo irradiated have increased permeability of their membrane. Due to this, potassium leaks from their membrane and the free potassium and hemoglobin in the unit of blood increases (103). Following irradiation, the expiration of RBC units is changed to 28 days post-irradiation. The original expiration date is kept if it is within the 28-day window.
In addition to irradiation, newer pathogen reduction technology is capable of inactivating white blood cells, as well as pathogens, and mitigating the risk of developing TA-GvHD. One such process is the INTERCEPT (Cerus Corporation, Concord, CA, USA) system, which is approved by the US Food and Drug Administration (FDA) for pathogen reduction of platelet components. This employs a psoralen, amotosalen, and ultraviolet (UVA) light. After treatment with amotosalen and exposure to UVA light, nucleic acids in the donor white blood cells are crosslinked and unable to replicate. Some studies have found an increased rate of alloimmunization with INTERCEPT-treated platelets and a need for more platelet transfusions owing to decreased in vivo platelet recovery. In Europe, a version is being used that utilizes riboflavin in place of amotosalen, Mirasol PRT System (Terumo BCT). This riboflavin treatment has been expanded to RBC units in addition to platelets.
Washed blood components
Washing blood components removes about 90–95% of plasma from the unit, as well as any additive solution that may be present. It also removes a great deal of cellular debris, white blood cells, and free hemoglobin, if present (104). Washing of cellular components is mainly done for patients who have severe allergic reaction to blood transfusions, to mitigate hyperkalemia due to free hemoglobin and to mitigate TRALI (104). For those who have severe allergic reactions, washing of cellular components can remove plasma proteins from the supernatant to eliminate the risk of reaction. This is recommended for those who have serious allergic reactions regardless of whether due to IgA in the unit or non-specific plasma proteins (104).
In patients who are at risk for hyperkalemia, washing RBC units can remove free hemoglobin from the unit of blood. This will prevent the risk of hyperkalemia in the patient. This can also be done with units of blood that are at or close to their expiration date to prevent potassium from affecting the recipient.
Lastly, some hospitals use washing to mitigate HLA sensitization in patients awaiting transplants. In addition to leukoreduction, washing RBC units has the potential to remove some of the unwanted white blood cells in the unit. Washing prior to transfusion can mitigate HLA sensitization in transplant recipience, which will then reduce the risk of rejection from HLA sensitization.
Interestingly, Wirtz et al. published a study titled “Washing or filtering of blood products does not improve outcome in a rat model of trauma and multiple transfusion”. The study was designed to determine whether or not optimizing (i.e., washing and or filtering blood products) blood would improve post transfusion outcome in trauma models. It was thought that removing white cells, storage lesion products (i.e., hemoglobin and extracellular vesicles), and additive would improve outcome post transfusion. What they found was that the optimized blood product group resulted in tissue damage and organ failure similar to the control group of non-optimized blood product (105). This challenges the efficacy of washing blood products for patients for anything other than allergic transfusion reactions. In addition to this study, the TOTAL study showed that transfusion of stored blood in critically ill pediatric patients improved oxygen perfusion by measurement of lactic acid concentration, without causing a hyperkalemic effect (106).
Volume-reduced blood components (with or without saline replacement)
Volume reduced products are indicated for those who need a large amount of product, for instance platelets, but are unable to receive a large volume. Patient populations that benefit from volume reduction include pediatric patients, neonatal patients, and patients at risk for circulatory overload such as congestive heart failure patients.
RBCs can be volume reduced to remove the supernatant from the container. They can also be split into multiple aliquots and transfused separately, over a larger amount of time. This has the potential to mitigate the risk if volume overload for the patient and allows them to receive their product without complications.
Platelets can be volume reduced to remove plasma or placed in platelet additive solution (PAS). In a standard unit of apheresis platelets, there is 100% plasma or 35% plasma and 65% PAS. Reducing the amount of plasma also reduces the amount of potentially incompatible plasma being transfused into the recipient and may reduce the risk of allergic reactions (107). Platelets have a shelf-life of 5 to 7 days, resulting in a constant challenge with maintaining an adequate inventory to meet patients’ needs. Most transfusion services try to give type specific platelets, but supply constraints require that incompatible platelets are often administered. Volume reducing with or without saline replacement or use of PAS can lower the adverse effects of transfusing incompatible plasma.
Frozen-thawed-deglycerolized RBCs
Currently, the International Society for Blood Transfusion (ISBT) recognizes 42 human blood group systems. (ISBTWeb.org) (108). Each system can contain multiple different antigens composed of amino acid sequences or carbohydrate structures. To place this in perspective, the ABO blood group is one blood group system, which contains multiple antigens and subgroups. Patients, who lack certain high frequency antigens, or a combination of moderate frequency antigens, present a challenge in transfusion medicine regarding available compatible blood.
RBC units can be stored anywhere from 21 to 42 days at 1–6 °C, depending on the anticoagulant and the addition of additive solution. Advances in science have led to the cryopreservation of RBC units. These units are treated with controlled addition and removal of glycerol to protect the cells from osmotic lysis and to protect the transfusion recipient’s exposure to the chemical cryoprotectant. The units preserved with 40% glycerol can be stored for up to 10 years at ≤−65 °C. Upon thawing, the cryopreserved units are then deglycerolized and washed in a hypertonic 12% saline solution, followed by an isotonic 0.9% saline solution (109). If done in an open system, these red cell units expire 24 hours post thaw. There is a closed system that allows the expiration date to be extended to 14 days.
HLA-matched and crossmatched and antigen negative platelets
Ideally, a unit of single donor apheresis platelets should increase the recipient’s platelet count by 30,000–60,000/μL. If platelet transfusion fails to increase the recipient’s platelet count sufficiently, platelet refractory mechanisms should be investigated. There are generally two reasons for refractoriness: immune mediated and non-immune. Non-immune causes are mechanical causes, such as sepsis, bleeding, and splenomegaly, where platelets are being actively destroyed. Immune refractoriness is caused by antibodies to either platelet antigens or HLA.
Human platelets express HLA class I antigens and human platelet antigens (HPAs). In multiply transfused patients, such as oncology patients, repeated exposure can contribute to the development of HLA antibodies (110). This exposure can also contribute to developing antibodies to HPAs.
In patients with immune refractoriness, platelet and HLA antibody studies can be performed. If an antibody is identified, platelets lacking the specific antigen can be selected for the recipient. Alternatively, platelet crossmatching may be performed and crossmatch-compatible platelets may be transfused.
Certain platelet antibodies can pose a risk to neonates. Fetal and neonatal alloimmune thrombocytopenia (FNAIT), occurs when maternal antibodies against platelet antigens cross the placenta causing severe thrombocytopenia. FNAIT can cause life threatening bleeding, specifically in the brain of the fetus and neonate. This most commonly occurs with anti-HPA-1a but has occurred rarely with other platelet antibodies (111). Platelets negative for the platelet antibody should be selected for the neonate until the circulating antibody has cleared (111).
Conclusions
Although transfusions can be a lifesaving therapy in patients who are hemorrhaging or severely anemic, unnecessary transfusions expose patients to increased risk and cost. Given that most of the evidence supporting a restrictive transfusion strategy has been published in the past decade, PBM programs have only recently gained popularity (5). Adherence to transfusion guidelines should be an institutional priority at every medical center. Widespread compliance with guidelines will result in increased safety as well as cost savings for patients, payers, and medical centers, as well as preservation of the blood supply for patients who truly need transfusions (5).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Paul D. Mintz) for the series “Transfusion Therapy: Principles and Practices” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-21-70/coif). The series “Transfusion Therapy: Principles and Practices” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McCoy KL. The providence hospital blood conservation program. Transfusion 1962;2:3-6. [Crossref]
- Friedman BA, Burns TL, Schork MA. An analysis of blood transfusion of surgical patients by sex: a question for the transfusion trigger. Transfusion 1980;20:179-88. [Crossref] [PubMed]
- Guinn NR, Maxwell C. Encouraging single-unit transfusions: a superior patient blood management strategy? Transfusion 2017;57:1107-8. [Crossref] [PubMed]
- Wang JK, Klein HG. Red blood cell transfusion in the treatment and management of anaemia: the search for the elusive transfusion trigger. Vox Sang 2010;98:2-11. [Crossref] [PubMed]
- Sadana D, Pratzer A, Scher LJ, et al. Promoting High-Value Practice by Reducing Unnecessary Transfusions With a Patient Blood Management Program. JAMA Intern Med 2018;178:116-22. [Crossref] [PubMed]
- Frank SM, John DJ, Resar LMS. Development of a patient blood management program. In: Waters JH, Frank SM. editors. Patient Blood Management: Multidisciplinary Approaches to Optimizing Care. Bethesda: AABB Press, 2016.
- Gammon RR. The role of metrics in patient blood management. In: Fredrich N, Gammon RR, Richards CA, Tauer R. PBM Metrics. Bethesda: AABB Press, 2019.
- Klein HG, Hrouda JC, Epstein JS. Crisis in the Sustainability of the U.S. Blood System. N Engl J Med 2017;377:1485-8. [Crossref] [PubMed]
- Mulcahy AW, Kapinos KA, Briscombe B, et al. Toward a sustainable blood supply in the United States: an analysis of the current system and alternatives for the future. Santa Monica: Rand Corporation, 2016.
- US Department of Health and Human Services. Adequacy of the National Blood Supply: Report to Congress 2020. 2020. Available online: https://www.aabb.org/docs/default-source/default-document-library/positions/hhs-report-to-congress-on-the-adequacy-of-the-national-blood-supply.pdf?sfvrsn=aeea20ff_4 (Accessed August 24, 2021).
- Goel R, Chappidi MR, Patel EU, et al. Trends in Red Blood Cell, Plasma, and Platelet Transfusions in the United States, 1993-2014. JAMA 2018;319:825-7. [Crossref] [PubMed]
- Whitaker B, Rajbhandary S, Kleinman S, et al. Trends in United States blood collection and transfusion: results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion 2016;56:2173-83. [Crossref] [PubMed]
- Goodnough LT, Hollenhorst MA. Clinical decision support and improved blood use in patient blood management. Hematology Am Soc Hematol Educ Program 2019;2019:577-82. [Crossref] [PubMed]
- Hibbs SP, Nielsen ND, Brunskill S, et al. The impact of electronic decision support on transfusion practice: a systematic review. Transfus Med Rev 2015;29:14-23. [Crossref] [PubMed]
- Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet 2011;378:1396-407. [Crossref] [PubMed]
- Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol 2013;30:270-382. Erratum in: Eur J Anaesthesiol 2014;31:247. [Crossref] [PubMed]
- Mueller MM, Van Remoortel H, Meybohm P, et al. Patient Blood Management: Recommendations From the 2018 Frankfurt Consensus Conference. JAMA 2019;321:983-97. [Crossref] [PubMed]
- Triphaus C, Judd L, Glaser P, et al. Effectiveness of Preoperative Iron Supplementation in Major Surgical Patients With Iron Deficiency: A Prospective Observational Study. Ann Surg 2021;274:e212-9. [Crossref] [PubMed]
- Ng O, Keeler BD, Mishra A, et al. Iron therapy for preoperative anaemia. Cochrane Database Syst Rev 2019;12:CD011588. [PubMed]
- Carson JL, Guyatt G, Heddle NM, et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA 2016;316:2025-35. [Crossref] [PubMed]
- National Institute for Health and Care Excellence. Blood Transfusion: NICE Guideline. 2015. Available online: https://www.nice.org.uk/guidance/ng24/resources/blood-transfusion-pdf-1837331897029 (Accessed October 11, 2021).
- McCormick M, Delaney M. Transfusion support: Considerations in pediatric populations. Semin Hematol 2020;57:65-72. [Crossref] [PubMed]
- Ferraris VA, Hochstetler M, Martin JT, et al. Blood transfusion and adverse surgical outcomes: The good and the bad. Surgery 2015;158:608-17. [Crossref] [PubMed]
- Klein AA, Collier T, Yeates J, et al. The ACTA PORT-score for predicting perioperative risk of blood transfusion for adult cardiac surgery. Br J Anaesth 2017;119:394-401. [Crossref] [PubMed]
- Klein AA, Bailey CR, Charlton AJ, et al. Association of Anaesthetists guidelines: cell salvage for peri-operative blood conservation 2018. Anaesthesia 2018;73:1141-50. [Crossref] [PubMed]
- Klein A, Agarwal S, Cholley B, et al. A survey of patient blood management for patients undergoing cardiac surgery in nine European countries. J Clin Anesth 2021;72:110311. [Crossref] [PubMed]
- Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471-82. [Crossref] [PubMed]
- Holcomb JB, del Junco DJ, Fox EE, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg 2013;148:127-36. [Crossref] [PubMed]
- Cowan T, Weaver N, Whitfield A, et al. The epidemiology of overtransfusion of red cells in trauma resuscitation patients in the context of a mature massive transfusion protocol. Eur J Trauma Emerg Surg 2021; Epub ahead of print. [Crossref] [PubMed]
- Jones JM, Sapiano MRP, Mowla S, et al. Has the trend of declining blood transfusions in the United States ended? Findings of the 2019 National Blood Collection and Utilization Survey. Transfusion 2021;61:S1-10. [Crossref] [PubMed]
- Savage W. Implementing a blood utilization program to optimize transfusion practice. Hematology Am Soc Hematol Educ Program 2015;2015:444-7. [Crossref] [PubMed]
- Saxena S. The Transfusion Committee: Membership, Organization, and Implementation. In: Saxena S. editor. The transfusion committee: Putting patient safety first. 2nd ed. Bethesda: AABB Press, 2013.
- Gammon RR. editor. Guide to Patient Blood Management and Blood Utilization. Bethesda: AABB Press, 2020.
- Frank SM, Oleyar MJ, Ness PM, et al. Reducing unnecessary preoperative blood orders and costs by implementing an updated institution-specific maximum surgical blood order schedule and a remote electronic blood release system. Anesthesiology 2014;121:501-9. [Crossref] [PubMed]
- Woo JS, Suslow P, Thorsen R, et al. Development and Implementation of Real-Time Web-Based Dashboards in a Multisite Transfusion Service. J Pathol Inform 2019;10:3. [Crossref] [PubMed]
- Ellrodt AG, Conner L, Riedinger M, et al. Measuring and improving physician compliance with clinical practice guidelines. A controlled interventional trial. Ann Intern Med 1995;122:277-82. [Crossref] [PubMed]
- Haspel RL, Uhl L. How do I audit hospital blood product utilization? Transfusion 2012;52:227-30. [Crossref] [PubMed]
- Gammon RR. Best Practice Alerts. In: Fredrich N, Gammon RR, Richards CA, et al. PBM Metrics. Bethesda: AABB Press, 2019.
- Thomas D, Thompson J, Ridler B. All Blood Counts: A manual for blood conservation and patient blood management. Shrewsbury: TFM Publishing Limited, 2016.
- Koch CG, Reineks EZ, Tang AS, et al. Contemporary bloodletting in cardiac surgical care. Ann Thorac Surg 2015;99:779-84. [Crossref] [PubMed]
- Shehata N, Mo YD. Hemotherapy decisions and their outcomes. In: Cohn CS, Delaney M, Johnson ST, et al. editors. Technical manual. 20th ed. Bethesda: AABB Press, 2020.
- Vuille-Lessard E, Boudreault D, Girard F, et al. Red blood cell transfusion practice in elective orthopedic surgery: a multicenter cohort study. Transfusion 2010;50:2117-24. [Crossref] [PubMed]
- Practice Guidelines for blood component therapy: A report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology 1996;84:732-47. [Crossref] [PubMed]
- Napolitano LM, Kurek S, Luchette FA, et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med 2009;37:3124-57. [Crossref] [PubMed]
- Bracey AW, Radovancevic R, Riggs SA, et al. Lowering the hemoglobin threshold for transfusion in coronary artery bypass procedures: effect on patient outcome. Transfusion 1999;39:1070-7. [Crossref] [PubMed]
- Bush RL, Pevec WC, Holcroft JW. A prospective, randomized trial limiting perioperative red blood cell transfusions in vascular patients. Am J Surg 1997;174:143-8. [Crossref] [PubMed]
- Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J 2013;165:964-71.e1. [Crossref] [PubMed]
- Carson JL, Sieber F, Cook DR, et al. Liberal versus restrictive blood transfusion strategy: 3-year survival and cause of death results from the FOCUS randomised controlled trial. Lancet 2015;385:1183-9. [Crossref] [PubMed]
- Carson JL, Terrin ML, Barton FB, et al. A pilot randomized trial comparing symptomatic vs. hemoglobin-level-driven red blood cell transfusions following hip fracture. Transfusion 1998;38:522-9. [Crossref] [PubMed]
- Carson JL, Terrin ML, Magaziner J, et al. Transfusion trigger trial for functional outcomes in cardiovascular patients undergoing surgical hip fracture repair (FOCUS). Transfusion 2006;46:2192-206. [Crossref] [PubMed]
- Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011;365:2453-62. [Crossref] [PubMed]
- Cooper HA, Rao SV, Greenberg MD, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study). Am J Cardiol 2011;108:1108-11. [Crossref] [PubMed]
- Fan YX, Liu FF, Jia M, et al. Comparison of restrictive and liberal transfusion strategy on postoperative delirium in aged patients following total hip replacement: a preliminary study. Arch Gerontol Geriatr 2014;59:181-5. [Crossref] [PubMed]
- Foss NB, Kristensen MT, Jensen PS, et al. The effects of liberal versus restrictive transfusion thresholds on ambulation after hip fracture surgery. Transfusion 2009;49:227-34. [Crossref] [PubMed]
- Gregersen M, Borris LC, Damsgaard EM. Postoperative blood transfusion strategy in frail, anemic elderly patients with hip fracture: the TRIFE randomized controlled trial. Acta Orthop 2015;86:363-72. [Crossref] [PubMed]
- Gregersen M, Borris LC, Damsgaard EM. Blood transfusion and overall quality of life after hip fracture in frail elderly patients--the transfusion requirements in frail elderly randomized controlled trial. J Am Med Dir Assoc 2015;16:762-6. [Crossref] [PubMed]
- Gregersen M, Damsgaard EM, Borris LC. Blood transfusion and risk of infection in frail elderly after hip fracture surgery: the TRIFE randomized controlled trial. Eur J Orthop Surg Traumatol 2015;25:1031-8. [Crossref] [PubMed]
- Grover M, Talwalkar S, Casbard A, et al. Silent myocardial ischaemia and haemoglobin concentration: a randomized controlled trial of transfusion strategy in lower limb arthroplasty. Vox Sang 2006;90:105-12. [Crossref] [PubMed]
- Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304:1559-67. [Crossref] [PubMed]
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409-17. Erratum in: N Engl J Med 1999;340:1056. [Crossref] [PubMed]
- Hébert PC, Wells G, Marshall J, et al. Transfusion requirements in critical care. A pilot study. Canadian Critical Care Trials Group. JAMA 1995;273:1439-44. Erratum in: JAMA 1995;274:944. [Crossref] [PubMed]
- Holst LB, Haase N, Wetterslev J, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med 2014;371:1381-91. [Crossref] [PubMed]
- Jairath V, Kahan BC, Gray A, et al. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (TRIGGER): a pragmatic, open-label, cluster randomised feasibility trial. Lancet 2015;386:137-44. [Crossref] [PubMed]
- Johnson RG, Thurer RL, Kruskall MS, et al. Comparison of two transfusion strategies after elective operations for myocardial revascularization. J Thorac Cardiovasc Surg 1992;104:307-14. [Crossref] [PubMed]
- Lacroix J, Hébert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med 2007;356:1609-19. [Crossref] [PubMed]
- Lotke PA, Barth P, Garino JP, et al. Predonated autologous blood transfusions after total knee arthroplasty: immediate versus delayed administration. J Arthroplasty 1999;14:647-50. [Crossref] [PubMed]
- Murphy GJ, Pike K, Rogers CA, et al. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med 2015;372:997-1008. Erratum in: N Engl J Med 2015;372:2274. [Crossref] [PubMed]
- Nielsen K, Johansson PI, Dahl B, et al. Perioperative transfusion threshold and ambulation after hip revision surgery--a randomized trial. BMC Anesthesiol 2014;14:89. [Crossref] [PubMed]
- Parker MJ. Randomised trial of blood transfusion versus a restrictive transfusion policy after hip fracture surgery. Injury 2013;44:1916-8. [Crossref] [PubMed]
- Mo A, Stanworth SJ, Shortt J, et al. Red cell transfusions: Is less always best?: How confident are we that restrictive transfusion strategies should be the standard of care default transfusion practice? Transfusion 2021;61:2195-203. [Crossref] [PubMed]
- Callum JL, Waters JH, Shaz BH, et al. The AABB recommendations for the Choosing Wisely campaign of the American Board of Internal Medicine. Transfusion 2014;54:2344-52. [Crossref] [PubMed]
- Podlasek SJ, Thakkar RN, Rotello LC, et al. Implementing a "Why give 2 when 1 will do?" Choosing Wisely campaign. Transfusion 2016;56:2164. [Crossref] [PubMed]
- Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205-13. [Crossref] [PubMed]
- Nahirniak S, Slichter SJ, Tanael S, et al. Guidance on platelet transfusion for patients with hypoproliferative thrombocytopenia. Transfus Med Rev 2015;29:3-13. [Crossref] [PubMed]
- Wandt H, Frank M, Ehninger G, et al. Safety and cost effectiveness of a 10×109/L trigger for prophylactic platelet transfusions compared with the traditional 20×109/L trigger: a prospective comparative trial in 105 patients with acute myeloid leukemia. Blood 1998;91:3601-6. [Crossref] [PubMed]
- Slichter SJ, Harker LA. Thrombocytopenia: mechanisms and management of defects in platelet production. Clin Haematol 1978;7:523-39. [Crossref] [PubMed]
- Heckman KD, Weiner GJ, Davis CS, et al. Randomized study of prophylactic platelet transfusion threshold during induction therapy for adult acute leukemia: 10,000/microL versus 20,000/microL. J Clin Oncol 1997;15:1143-9. [Crossref] [PubMed]
- Rebulla P, Finazzi G, Marangoni F, et al. The threshold for prophylactic platelet transfusions in adults with acute myeloid leukemia. Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto. N Engl J Med 1997;337:1870-5. [Crossref] [PubMed]
- Zumberg MS, del Rosario ML, Nejame CF, et al. A prospective randomized trial of prophylactic platelet transfusion and bleeding incidence in hematopoietic stem cell transplant recipients: 10,000/L versus 20,000/microL trigger. Biol Blood Marrow Transplant 2002;8:569-76. [Crossref] [PubMed]
- Schiffer CA, Bohlke K, Delaney M, et al. Platelet Transfusion for Patients With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2018;36:283-99. [Crossref] [PubMed]
- Slichter SJ, Kaufman RM, Assmann SF, et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med 2010;362:600-13. [Crossref] [PubMed]
- Damiani G, Pinnarelli L, Sommella L, et al. Appropriateness of fresh-frozen plasma usage in hospital settings: a meta-analysis of the impact of organizational interventions. Transfusion 2010;50:139-44. [Crossref] [PubMed]
- Roback JD, Caldwell S, Carson J, et al. Evidence-based practice guidelines for plasma transfusion. Transfusion 2010;50:1227-39. [Crossref] [PubMed]
- Costa-Filho R, Hochleitner G, Wendt M, et al. Over 50 Years of Fibrinogen Concentrate. Clin Appl Thromb Hemost 2016;22:109-14. [Crossref] [PubMed]
- Yang L, Stanworth S, Baglin T. Cryoprecipitate: an outmoded treatment? Transfus Med 2012;22:315-20. [Crossref] [PubMed]
- Callum JL, Karkouti K, Lin Y. Cryoprecipitate: the current state of knowledge. Transfus Med Rev 2009;23:177-88. [Crossref] [PubMed]
- Bezinover D, Dirkmann D, Findlay J, et al. Perioperative Coagulation Management in Liver Transplant Recipients. Transplantation 2018;102:578-92. [Crossref] [PubMed]
- Arya RC, Wander G, Gupta P. Blood component therapy: Which, when and how much. J Anaesthesiol Clin Pharmacol 2011;27:278-84. [Crossref] [PubMed]
- Strauss RG. Role of granulocyte/neutrophil transfusions for haematology/oncology patients in the modern era. Br J Haematol 2012;158:299-306. [Crossref] [PubMed]
- Strauss RG. Granulocyte (Neutrophil) Transfusion. In: McLeod BC, Szczepiorkowski ZM, Weinstein R, et al. editors. Apheresis: Principles and Practice. 3rd ed. Bethesda: AABB Press, 2010.
- Gammon RR. editor. Standards for blood banks and transfusion services. 32nd ed. Bethesda: AABB Press, 2020.
- Gilliss BM, Looney MR, Gropper MA. Reducing noninfectious risks of blood transfusion. Anesthesiology 2011;115:635-49. [Crossref] [PubMed]
- Shapiro MJ. To filter blood or universal leukoreduction: what is the answer? Crit Care 2004;8:S27-30. [Crossref] [PubMed]
- Sharma RR, Marwaha N. Leukoreduced blood components: Advantages and strategies for its implementation in developing countries. Asian J Transfus Sci 2010;4:3-8. [Crossref] [PubMed]
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Biologics Evaluation and Research. Guidance for Industry: Pre-Storage Leukocyte Reduction of Whole Blood and Blood Components Intended for Transfusion. 2012. Available online: https://www.fda.gov/media/84460/download (Accessed August 25, 2021).
- Richter JR, Sutton JM, Hexley P, et al. Leukoreduction of packed red blood cells attenuates proinflammatory properties of storage-derived microvesicles. J Surg Res 2018;223:128-35. [Crossref] [PubMed]
- Blajchman MA, Goldman M, Freedman JJ, et al. Proceedings of a consensus conference: prevention of post-transfusion CMV in the era of universal leukoreduction. Transfus Med Rev 2001;15:1-20.
- Vamvakas EC. Is white blood cell reduction equivalent to antibody screening in preventing transmission of cytomegalovirus by transfusion? A review of the literature and meta-analysis. Transfus Med Rev 2005;19:181-99. [Crossref] [PubMed]
- Bowden RA, Slichter SJ, Sayers M, et al. A comparison of filtered leukocyte-reduced and cytomegalovirus (CMV) seronegative blood products for the prevention of transfusion-associated CMV infection after marrow transplant. Blood 1995;86:3598-603. [Crossref] [PubMed]
- Josephson CD, Caliendo AM, Easley KA, et al. Blood transfusion and breast milk transmission of cytomegalovirus in very low-birth-weight infants: a prospective cohort study. JAMA Pediatr 2014;168:1054-62. [Crossref] [PubMed]
- Foukaneli T, Kerr P, Bolton-Maggs PHB, et al. Guidelines on the use of irradiated blood components. Br J Haematol 2020;191:704-24. [Crossref] [PubMed]
- FDA. Recommendations regarding license amendment and procedures for gamma irradiation of blood products. 1993. Available online: https://www.fda.gov/media/70934/download (Accessed August 25, 2021).
- Adams F, Bellairs G, Bird AR, et al. Biochemical storage lesions occurring in nonirradiated and irradiated red blood cells: a brief review. Biomed Res Int 2015;2015:968302. [Crossref] [PubMed]
- Cardigan R, New HV, Tinegate H, et al. Washed red cells: theory and practice. Vox Sang 2020;115:606-16. [Crossref] [PubMed]
- Wirtz MR, Jurgens J, Zuurbier CJ, et al. Washing or filtering of blood products does not improve outcome in a rat model of trauma and multiple transfusion. Transfusion 2019;59:134-45. [Crossref] [PubMed]
- Dhabangi A, Ainomugisha B, Cserti-Gazdewich C, et al. Effect of Transfusion of Red Blood Cells With Longer vs Shorter Storage Duration on Elevated Blood Lactate Levels in Children With Severe Anemia: The TOTAL Randomized Clinical Trial. JAMA 2015;314:2514-23. [Crossref] [PubMed]
- Cohn CS, Stubbs J, Schwartz J, et al. A comparison of adverse reaction rates for PASC versus plasma platelet units. Transfusion 2014;54:1927-34. [Crossref] [PubMed]
- ISBTWeb.org. Red Cell Immunogenetics and Blood Group Terminology. Available online: https://www.isbtweb.org/working-parties/red-cell-immunogenetics-and-blood-group-terminology (Accessed September 18, 2021).
- Cap A, Badloe J, Woolley T, et al. The Use of Frozen and Deglycerolized Red Blood Cells. Mil Med 2018;183:52-4. [Crossref] [PubMed]
- Pena JR, Eisenbrey AB, Kopko PM. The HLA System. In: Cohn CS, Delaney M, Johnson ST, et al. editors. Technical Manual. 20th ed. Bethesda: AABB Press, 2020.
- Regan F, Lees CC, Jones B, et al. Prenatal Management of Pregnancies at Risk of Fetal Neonatal Alloimmune Thrombocytopenia (FNAIT): Scientific Impact Paper No. 61. BJOG 2019;126:e173-85. [Crossref] [PubMed]
Cite this article as: Gammon RR, Coberly E, Dubey R, Jindal A, Nalezinski S, Varisco JL. Patient blood management—it is about transfusing blood appropriately. Ann Blood 2022;7:21.


