
Congenital and acquired disorders of primary hemostasis
Introduction
Many congenital and acquired disorders of primary hemostasis may result in serious clinical disease requiring subspecialty care by a hematologist. With advancements in understanding of the molecular underpinnings and relationships within primary hemostasis, clinical testing and treatment paradigms continue to evolve and become increasingly complex, requiring multidisciplinary care and expert clinical laboratories. While not exhaustive, this review attempts to outline and provide insight into congenital and acquired disorders of primary hemostasis. Specifically, it will focus on qualitative disorders of primary hemostasis, except those that predominantly manifest with thrombocytopenia and/or are associated with clinical thrombosis (e.g., thrombotic thrombocytopenic purpura and heparin-induced thrombocytopenia, for instance).
Congenital
Von Willebrand disease (VWD)
VWD is a heterogeneous mucocutaneous bleeding disorder with a worldwide incidence of 1–2%; it may be caused by quantitative (Types 1 and 3) or qualitative (Type 2) abnormalities of von Willebrand factor (VWF) (1). VWD Type 1 is a mild deficiency of VWF and the most common, affecting approximately 80% of patients with VWD, while Type 3 is a severe deficiency of VWD (2). Type 2 has several subtypes, each with unique qualitative defects. VWF promotes platelet adhesion to injured arterial or arteriolar walls, which is why its deficiency causes platelet-type bleeding. Since VWF is also the carrier molecule for factor VIII (FVIII), those with Type 3 VWD have low FVIII activity as it has a plasma half-life of seconds when not bound to VWF. Therefore, in addition to mucocutaneous bleeding, patients with Type 3 VWD also experience soft-tissue (hemophilia-like) bleeding.
Unlike other coagulation factor deficiencies, diagnosis and classification of VWD require several laboratory assays (Table 1). In Types 1 and 3, since there is only a deficiency of VWF (no functional defect), the results of the VWF activity and the antigen assays are similar (ratio ≥0.7), while in Type 2 (except 2N), the ratio is low due to a qualitative defect of VWF. The FVIII level more closely parallels the VWF antigen than the VWF activity in all types, except Type 2N. Although not considered part of a “VWD profile”, the patient’s blood group is important to interpret VWF results: healthy blood donors of blood group O and A were found to have VWF activity as low as 36% and 48%, respectively, which are below the commonly published reference interval of 50–166% (3). Consequently, individuals of blood group O or A could have a false positive diagnosis of VWD. Conversely, the lower end of the reference range for those of AB blood group is 64%, increasing their chance of having the diagnosis of VWD missed if the VWF activity is between 50–63%. Thus, a disproportionate number of people with blood group O have “low VWF” (levels of 30–50%), which may be associated with mild intermittent mucocutaneous bleeding. With advancements in understanding VWD pathogenesis and increasingly complex laboratory assays, management has also become more complex (4). In Type 1 VWD, there may be relatively adequate levels of VWF stored in the Weibel-Palade bodies of endothelial cells that can be released into the plasma with desmopressin (DDAVP) (Figure 1). To ensure a patient’s response, a DDAVP challenge is commonly used, in which the baseline VWF antigen and activity levels are measured, DDAVP is given, and they are measured again hourly to follow the peak and subsequent plasma clearance of VWF. If the level rises appropriately, DDAVP may be used for bleeding or prior to procedures; if not, a VWF concentrate is required. In subtype 2B, DDAVP is contraindicated because the released VWF with increased affinity for its platelet receptor may cause thrombocytopenia. In subtype 2N and Type 3, a concentrate of VWF and FVIII is the preferred treatment. The most commonly available VWF concentrates are plasma-derived, although a recombinant form has been approved by the Food and Drug Administration (FDA). Although rich in VWF, cryoprecipitate should only be used in situations where no other preparation is available, as blood products confer a risk of infectious disease transmission.
Table 1
| Type | Pathophysiology | Bleeding | VWD profile | Ancillary tests |
|---|---|---|---|---|
| 1 | Various dominant mutations throughout VWF gene | Mild to moderate mucocutaneous | VWF:Ag, VWF:RCo, and FVIII activity <30%; VWF:Ag/VWF:RCo ratio ≥0.7 | None |
| 2A | Dominant or recessive mutation in ADAMTS13 cleavage site of VWF | Moderate to severe mucocutaneous | VWF:Ag and FVIII may be normal or moderately reduced; VWF:RCo <30%; VWF:Ag/VWF:RCo ratio <0.7 | VWF multimer analysis with absence of large multimers |
| 2B | Dominant gain of function mutation in GPIb/IX/V binding site of VWF | Mild to moderate mucocutaneous | VWF:Ag and FVIII may be normal or moderately reduced; VWF:RCo <30%; VWF:Ag/VWF:RCo ratio <0.7 | VWF multimer analysis absence of large and intermediate-size multimers Ristocetin response curve: agglutination at ristocetin concentrations <1 mg/mL |
| 2M | Dominant or recessive loss of function mutation in GPIb/IX/V binding site of VWF | Moderate mucocutaneous | VWF:Ag and FVIII may be normal or moderately reduced; VWF:RCo <30%; VWF:Ag/VWF:RCo ratio <0.7 | Normal VWF multimer analysis |
| 2N | Recessive mutation in FVIII binding site of VWF | Mild soft tissue | VWF:Ag and VWF:RCo normal; FVIII activity <20%; VWF:Ag/VWF:RCo ratio ≥0.7 | Normal VWF multimer analysis |
| 3 | Recessive deletion with decreased mRNA expression | Severe mucocutaneous and soft tissue | VWF:Ag, VWF:RCo and FVIII activity undetectable; VWF:Ag/VWF:RCo ratio cannot be calculated | None |
VWD, von Willebrand disease; VWF, von Willebrand factor; ADAMTS13, a disintegrin-like and metalloprotease domain with thrombospondin motifs-13; VWF:Ag, von Willebrand factor antigen; VWF:RCo, von Willebrand factor ristocetin cofactor assay (VWF activity); GP, glycoprotein.
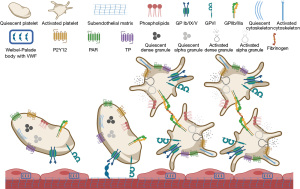
Disorders of platelet receptors
Bernard-Soulier syndrome (BSS)
BSS is a rare congenital platelet function disorder. It results from abnormalities in the platelet GPIb/IX/V complex, which mediates the binding of VWF to platelets, especially at high shear rates (Figure 1). In BSS, the platelet count is moderately decreased, and platelets are large (mean platelet volume between 14.6 and 16.9 fL) (5-7). BSS results from mutations in GP1BA (17p13), GPIBB (22q11), and GP9 (3q21), which inhibit appropriate function of the GPIb/IX/V complex necessary for platelet adhesion to VWF. Disease can be severe or moderate based on biallelic or monoallelic abnormalities, respectively (5-7). Flow cytometry shows decreased platelet surface expression of GPIb (CD42) and is the definitive diagnostic test. Platelet aggregometry studies show normal responses to ADP, epinephrine, thrombin, and collagen, but not to ristocetin. With the latter, there is either decreased or absent platelet agglutination, making BSS indistinguishable from VWD. Unlike in VWD, however, plasma VWF antigen and FVIII activity are normal (6,8). Antifibrinolytics alone or in combination with other measures (e.g., nasal packing for nosebleed, hormonal treatment for menstrual bleeding) are first-line approaches. Platelet transfusion and recombinant factor VIIa may be used for more severe bleeding, although the latter is not approved by the FDA for this indication. Hematopoietic stem cell transplant may be an option for recurrent severe bleeding or patients with GPIb/IX/V antibodies (8).
Glanzmann thrombasthenia (GT)
GT is a rare autosomal recessive disorder characterized by markedly impaired platelet aggregation and severe mucocutaneous bleeding. Unlike in BSS, platelet number and appearance are unaffected (5-7). Pathology results from mutations in ITGA2B and/or ITGB3, both located on chromosome 17, which cause quantitative or qualitative defects in the GPIIb/IIIa complex (5-7). Platelet aggregometry studies show lack or markedly decreased response to all platelet agonists (except ristocetin), with absence of both the primary and secondary waves of aggregation. The diagnosis can be confirmed by demonstrating greatly decreased or absent platelet expression of the GPIIb/IIIa (CD41 or CD61) complex by flow cytometry (6-8). As in BSS, antifibrinolytics alone and/or local measures are first-line in management of bleeding. Platelet transfusions and recombinant factor VIIa may be used for more intense bleeding, which is FDA approved for patients with refractoriness to platelet transfusions, with or without antibodies to GPIIb/IIIa. Hematopoietic stem cell transplant may be an option for recurrent severe bleeding or for patients with GPIIb/IIIa antibodies (8).
Platelet-type VWD (PT-VWD)
Primary hemostasis depends on a malleable bond between the platelet surface glycoprotein Ib alpha (GPIbα) and the A1 domain of VWF (Figure 1) (9). PT-VWD is caused by gain of function mutations in GP1BA, which leads to excessive binding between platelets and VWF with resultant clearance of this complex, causing thrombocytopenia and loss of high molecular weight VWF multimers (10,11). It is a rare autosomal dominant bleeding disorder similar to Type 2B VWD (12). Therefore, patients with a provisional diagnosis of Type 2B VWD or unexplained thrombocytopenia should be evaluated for PT-VWD with ristocetin-induced platelet aggregation (RIPA) mixing studies followed by genetic analysis if indicated, to confirm the diagnosis of PT-VWD (13,14). In cases of major bleeding, platelet transfusion with VWF-rich concentrates (if VWF is also low) is recommended. If there is no response to platelet transfusion or with severe bleeding, recombinant factor VIIa (90 µg/kg every 2 hours, as needed) with or without VWF-rich concentrates is useful (14). The dose of VWF-rich concentrate depends on the VWF activity in the plasma. For minor bleeding episodes, antifibrinolytics may be used. DDAVP should only be considered if a DDAVP trial has been completed and demonstrated appropriate rise in VWF antigen and activity levels. For pregnant patients with PT-VWD, checking platelet count and VWF activity in the first antenatal visit, once per trimester, at term, and at 2–4 weeks post-delivery are recommended (14).
GPVI deficiency
Platelets interact directly with collagen through specific receptors, such as GPVI (Figure 1) (15,16). Little is known about the genetic basis of GPVI deficiency, but its interaction with collagen activates signaling pathways leading to platelet activation and aggregation (17). There are case reports of patients with acquired and inherited deficiencies of GPVI presenting with mild bleeding and thrombocytopenia (18,19). These patients have abnormal platelet aggregation in response to collagen and other GPVI agonists. Since patients with GPVI defects are rare and may have only a mild bleeding tendency, standard clinical presentation and treatment are poorly defined (20). In cases with autoantibodies to GPVI and bleeding, corticosteroids have been used (18,19).
Storage pool deficiencies
Platelets contain a diverse array of biochemically active substances in order to perform their physiologic roles. Many of them are stored in intracellular organelles, primarily alpha and dense granules (Figure 1). Contents of the alpha granules include platelet-derived growth factor, platelet factor 4, VWF, fibrinogen, and FV; whereas dense granules contain serotonin, calcium, adenosine diphosphate (ADP), and adenosine triphosphate (ATP). Disorders of primary hemostasis can arise when there are defects of these granules and their contents, collectively known as “storage pool deficiencies”. These often appear in the setting of other syndromes. Storage pool deficiencies are classically diagnosed using multi-method approaches. If platelet aggregometry is used, absence of a “second wave” is characteristic, and the diagnosis should be confirmed by electron microscopy. Diagnostic testing continues to advance with the use of flow cytometry and genetic evaluation. These conditions are relatively rare and treatment plans are individualized. Often, DDAVP and/or antifibrinolytics are tried for bleeding episodes or prophylaxis for upcoming procedures, in an attempt to avoid platelet transfusions, thus minimizing the risk of alloimmunization. If these measures do not control bleeding, additional options such as recombinant factor VIIa may be needed. The most common storage pool deficiencies each have unique clinical presentations and features (Table 2).
Table 2
| Diagnosis | Notes |
|---|---|
| Alpha granule deficiencies | |
| Gray platelet syndrome | Gray appearance (light microscopy) |
| Macrothrombocytopenia | |
| Splenomegaly | |
| Myelofibrosis | |
| Variable inheritance | |
| -Autosomal recessive (NBEAL2) | |
| -Autosomal dominant (GFI1b) | |
| -X-linked (GATA1) | |
| Quebec platelet disorder | Autosomal dominant (PLAU) |
| Increased granule proteolysis | |
| Decreased factor V | |
| Delayed bleeding | |
| Dense granule deficiencies | |
| Hermansky-Pudlak syndrome | Oculocutaneous albinism |
| Autosomal recessive (multiple genes) | |
| Pulmonary fibrosis | |
| Granulomatous colitis | |
| Relatively prevalent in Puerto Rico | |
| Chediak-Higashi syndrome | Oculocutaneous albinism |
| Autosomal recessive (LYST) | |
| Immunodeficiency | |
| Neurologic manifestations | |
| Cytoplasmic inclusions | |
| Hemophagocytic lymphohistiocytosis |
Acquired
Qualitative platelet disorders due to medications
Medications to manage patients with atherosclerosis are designed to inhibit platelet activation to prevent arterial thrombosis. However, drugs intended for other purposes can also interfere with platelet function as an unintended side-effect. In the latter category, the majority have demonstrated in vitro antiplatelet effects without clear clinical consequences, with few having caused documented clinically relevant bleeding (Table 3) (21-25). Drugs for the treatment and prevention of arterial thrombosis inhibit platelets via multiple mechanisms, including cyclooxygenase pathway (aspirin), P2Y12 receptor (clopidogrel, prasugrel, ticagrelor), and integrin GPIIb/IIIa antagonism (abciximab, eptifibatide, tirofiban) (Table 3). Platelet aggregometry testing is the primary diagnostic test to identify medication-induced platelet dysfunction. The Platelet Function Analyzer (PFA)-100 is a rapid, automated assay that can be used to screen for abnormalities in primary hemostasis but does not provide detail on the mechanism of an identified deficit (26). The PFA-100 and other assays (e.g., VerifyNow, thromboelastogram (TEG), PlateletMapping, INNOVANCE PFA-200 system) have been used to monitor response to anti-platelet therapy, although available data do not support their routine use for identification of so-called aspirin or clopidogrel “resistance” (27). Medication cessation is an essential first step in cases of bleeding. The utility of platelet transfusion is debated, with limited studies available to evaluate safety and efficacy (28). The Platelet Transfusion in Cerebral Hemorrhage (PATCH) trial demonstrated worse outcomes in patients on anti-platelet therapy presenting with non-traumatic intracranial hemorrhage who received transfusion compared to those who did not (29). Uncertainty remains around the role of platelet transfusion for patients on P2Y12 inhibitors versus aspirin prior to emergent surgical interventions (30,31). One study found platelet transfusion did not restore platelet function in patients being treated with clopidogrel, but was efficient for patients being treated with aspirin (30).
Table 3
| Category | Medication | Mechanism of action | Notes |
|---|---|---|---|
| Intended antiplatelet effects | Aspirin (21) | Irreversible inhibition of COX-1 | Short half-life (15–20 minutes) but antiplatelet effect persists for the platelet lifespan (8–10 days) |
| P2Y12 receptor inhibitors (22) | Inhibition of ADP-induced platelet aggregation | Includes: clopidogrel, ticlopidine, prasugrel. Variable properties including time to onset, duration of effect | |
| Integrin αIIbβ3 antagonist (23) | Inhibits platelet interaction between GPIIb/IIIa and fibrinogen | Includes: abciximab, eptifibatide, tirofiban | |
| PDE inhibitors (24) | Increase hydrolysis of cAMP and cGMP | PDE5i: dypiridamole; PDE3i: cilostazol | |
| Unintended antiplatelet effects | Non-aspirin NSAIDs | Reversible inhibition of COX-1 | Short duration of effect. Certain NSAIDs are selective for COX-2 and do not have effects on platelet function |
| Beta-lactam antibiotics | Irreversible inhibition of agonist receptors | Specific antibiotic, dose, and duration of use all influence antiplatelet effect | |
| SSRIs | Decreased intra-platelet calcium mobilization; lower expression of multiple surface proteins | Increased bleeding risk observed with all medications in this category (e.g., fluoxetine, paroxetine, sertraline, citalopram) | |
| PDE5 inhibitors | Increase hydrolysis of cGMP | Includes: sildenafil, tadalafil, vardenafil |
COX, cyclooxygenase; ADP, adenosine diphosphate; VWF, von Willebrand factor; cAMP, cyclic adenosine 3',5'-monophosphate; cGMP, cyclic guanosine 3',5'-monophosphate; PDE, phosphodiesterase; NSAIDs, non-steroidal anti-inflammatory drugs; SSRIs, selective serotonin reuptake inhibitors.
Platelet dysfunction in systemic diseases
Liver disease
Liver disease is commonly associated with thrombocytopenia secondary to splenic sequestration and decreased levels of thrombopoietin. While these may contribute to abnormal primary hemostasis, qualitative disorders have also been described. Observations include GPIb abnormalities, storage pool deficiencies, and secretory defects (32). The effects of liver disease on hemostasis are intricate and dynamic, often requiring management on an individual basis and involving supportive care while addressing the underlying liver disease and driving etiologies.
Renal disease
Platelet dysfunction in the setting of renal disease is complex and not well understood, but it is often attributed to the accumulation of toxic metabolites, including uremic toxins, and abnormal nitric oxide metabolism (32,33). These defects are thought to impair adhesion, aggregation, and secretory functions of platelets. In order to address the increased bleeding tendency of individuals with advanced renal disease, several stabilizing measures can be pursued. These include dialysis, DDAVP, cryoprecipitate, red blood cell transfusions to target a hemoglobin of 10 g/dL, and conjugated estrogens. Platelet transfusions are typically avoided for bleeding prophylaxis under the premise that they too would rapidly become dysfunctional. However, they may be given if there is active bleeding.
Malignancy
Cancer has the ability to interfere with hemostasis on many fronts. It can cause thrombosis and related sequelae, metastasize to the bone marrow and result in myelophthisis, cause liver and/or renal disease, require cytotoxic chemoimmunotherapies, lead to disseminated intravascular coagulation, predispose to systemic infections, and more (34). With cancers of the bone marrow, such as myeloproliferative neoplasms and leukemias, there can be relative decreases in platelet production coupled with systemic dilution from the malignant cell line, in addition to changes in the platelet precursors themselves. These disorders have also been linked to qualitative changes of platelets in vitro, such as aberrant morphology and cytoplasmic contents, but clinical correlation to bleeding phenotypes has yet to be established. Dysproteinemias have several unique interactions with hemostasis, including the potential for hyperviscosity, vascular abnormalities if amyloid is deposited, direct interaction and alteration of platelet surfaces by a paraprotein, and acquired VWD from paraproteins acting as autoantibodies to VWF (35). In addition to supportive care, the mainstay of treatment of malignancy-induced primary hemostasis defects is to address the underlying malignancy, with platelet transfusions being an option for thrombocytopenia causing bleeding.
Mechanical
Extracorporeal mechanical support of the cardiovascular system is increasingly implemented in clinical practice, especially in the era of COVID-19. From ventricular assist devices and cardiac bypass machines to extracorporeal membrane oxygenation circuits, blood is pumped through artificial devices (36,37). Given the high flow rates and non-physiological surfaces, in addition to the required use of anticoagulation, these circuits can lead to disorders of primary hemostasis. Conceptualized as an iatrogenic Heyde’s syndrome, the turbulent flow generated by such devices can also lead to acquired VWD by shear-stress induced cleavage of large multimers of VWF or increasing its susceptibility to ADAMTS13 (a disintegrin-like and metalloprotease domain with thrombospondin motifs-13) enzymatic degradation. Additionally, platelets become activated when in contact with non-physiologic material and may develop acquired storage pool deficiencies. Moreover, in the setting of decreased fibrinogen, these mechanical circuits create complex hemostatic profiles. For these reasons, approaches to treatment are highly individualized and depend on the clinical situation. This is a field of hemostasis in need of further research and understanding in order to optimize and standardize management. As technologies evolve, including fibrinogen concentrates, pathogen-reduced cryoprecipitate, and alternative anticoagulation strategies, the hemostatic problems imbued by such mechanical devices will require interdisciplinary efforts to remedy (38,39).
Acknowledgments
The authors would like to thank all individuals living with and/or caring for those with disorders of hemostasis.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Paul D. Mintz) for the series “Transfusion Therapy: Principles and Practices” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/aob-21-77/coif). The series “Transfusion Therapy: Principles and Practices” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nichols WL, Hultin MB, James AH, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia 2008;14:171-232. [Crossref] [PubMed]
- James PD, Connell NT, Ameer B, et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv 2021;5:280-300. [Crossref] [PubMed]
- Gill JC, Endres-Brooks J, Bauer PJ, et al. The effect of ABO blood group on the diagnosis of von Willebrand disease. Blood 1987;69:1691-5. [Crossref] [PubMed]
- Connell NT, Flood VH, Brignardello-Petersen R, et al. ASH ISTH NHF WFH 2021 guidelines on the management of von Willebrand disease. Blood Adv 2021;5:301-25. [Crossref] [PubMed]
- Nurden AT, Nurden P. Inherited thrombocytopenias: history, advances and perspectives. Haematologica 2020;105:2004-19. [Crossref] [PubMed]
- Gresele PSubcommittee on Platelet Physiology of the International Society on Thrombosis and Hemostasis. Diagnosis of inherited platelet function disorders: guidance from the SSC of the ISTH. J Thromb Haemost 2015;13:314-22. [Crossref] [PubMed]
- Noris P, Pecci A. Hereditary thrombocytopenias: a growing list of disorders. Hematology Am Soc Hematol Educ Program 2017;2017:385-99. [Crossref] [PubMed]
- Grainger JD, Thachil J, Will AM. How we treat the platelet glycoprotein defects; Glanzmann thrombasthenia and Bernard Soulier syndrome in children and adults. Br J Haematol 2018;182:621-32. [Crossref] [PubMed]
- Fu H, Jiang Y, Yang D, et al. Flow-induced elongation of von Willebrand factor precedes tension-dependent activation. Nat Commun 2017;8:324. [Crossref] [PubMed]
- Othman M. Platelet-type Von Willebrand disease: three decades in the life of a rare bleeding disorder. Blood Rev 2011;25:147-53. [Crossref] [PubMed]
- Othman M, Emsley J. Gene of the issue: GP1BA gene mutations associated with bleeding. Platelets 2017;28:832-6. [Crossref] [PubMed]
- Enayat MS, Guilliatt AM, Lester W, et al. Distinguishing between type 2B and pseudo-von Willebrand disease and its clinical importance. Br J Haematol 2006;133:664-6. [Crossref] [PubMed]
- Favaloro EJ, Patterson D, Denholm A, et al. Differential identification of a rare form of platelet-type (pseudo-) von Willebrand disease (VWD) from Type 2B VWD using a simplified ristocetin-induced-platelet-agglutination mixing assay and confirmed by genetic analysis. Br J Haematol 2007;139:623-6. [Crossref] [PubMed]
- Othman M, Gresele P. Guidance on the diagnosis and management of platelet-type von Willebrand disease: A communication from the Platelet Physiology Subcommittee of the ISTH. J Thromb Haemost 2020;18:1855-8. [Crossref] [PubMed]
- Savage B, Saldívar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell 1996;84:289-97. [Crossref] [PubMed]
- Varga-Szabo D, Pleines I, Nieswandt B. Cell adhesion mechanisms in platelets. Arterioscler Thromb Vasc Biol 2008;28:403-12. [Crossref] [PubMed]
- Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood 2003;102:449-61. [Crossref] [PubMed]
- Nurden AT. Clinical significance of altered collagen-receptor functioning in platelets with emphasis on glycoprotein VI. Blood Rev 2019;38:100592. [Crossref] [PubMed]
- Arthur JF, Dunkley S, Andrews RK. Platelet glycoprotein VI-related clinical defects. Br J Haematol 2007;139:363-72. [Crossref] [PubMed]
- Jandrot-Perrus M, Hermans C, Mezzano D. Platelet glycoprotein VI genetic quantitative and qualitative defects. Platelets 2019;30:708-13. [Crossref] [PubMed]
- Patrono C. Aspirin as an antiplatelet drug. N Engl J Med 1994;330:1287-94. [Crossref] [PubMed]
- Wallentin L. P2Y(12) inhibitors: differences in properties and mechanisms of action and potential consequences for clinical use. Eur Heart J 2009;30:1964-77. [Crossref] [PubMed]
- Quinn MJ, Byzova TV, Qin J, et al. Integrin alphaIIbbeta3 and its antagonism. Arterioscler Thromb Vasc Biol 2003;23:945-52. [Crossref] [PubMed]
- Gresele P, Momi S, Falcinelli E. Anti-platelet therapy: phosphodiesterase inhibitors. Br J Clin Pharmacol 2011;72:634-46. [Crossref] [PubMed]
- George JN, Shattil SJ. The clinical importance of acquired abnormalities of platelet function. N Engl J Med 1991;324:27-39. [Crossref] [PubMed]
- Favaloro EJ. Clinical utility of the PFA-100. Semin Thromb Hemost 2008;34:709-33. [Crossref] [PubMed]
- Michelson AD, Bhatt DL. How I use laboratory monitoring of antiplatelet therapy. Blood 2017;130:713-21. [Crossref] [PubMed]
- Nagalla S, Sarode R. Role of platelet transfusion in the reversal of anti-platelet therapy. Transfus Med Rev 2019;33:92-7. [Crossref] [PubMed]
- Baharoglu MI, Cordonnier C, Al-Shahi Salman R, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet 2016;387:2605-13. [Crossref] [PubMed]
- Taylor G, Osinski D, Thevenin A, et al. Is platelet transfusion efficient to restore platelet reactivity in patients who are responders to aspirin and/or clopidogrel before emergency surgery? J Trauma Acute Care Surg 2013;74:1367-9. [Crossref] [PubMed]
- Godier A, Albaladejo POn Perioperative Haemostasis Gihp Group TFWG. Management of bleeding events associated with antiplatelet therapy: evidence, uncertainties and pitfalls. J Clin Med 2020;9:2318. [Crossref] [PubMed]
- Lambert MP. Platelets in liver and renal disease. Hematology Am Soc Hematol Educ Program 2016;2016:251-5. [Crossref] [PubMed]
- Boccardo P, Remuzzi G, Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost 2004;30:579-89. [Crossref] [PubMed]
- Kvolik S, Jukic M, Matijevic M, et al. An overview of coagulation disorders in cancer patients. Surg Oncol 2010;19:e33-46. [Crossref] [PubMed]
- Cuker A, Connors JM, Katz JT, et al. Clinical problem-solving. A bloody mystery. N Engl J Med 2009;361:1887-94. [Crossref] [PubMed]
- Murphy DA, Hockings LE, Andrews RK, et al. Extracorporeal membrane oxygenation-hemostatic complications. Transfus Med Rev 2015;29:90-101. [Crossref] [PubMed]
- Thomas J, Kostousov V, Teruya J. Bleeding and thrombotic complications in the use of extracorporeal membrane oxygenation. Semin Thromb Hemost 2018;44:20-9. [Crossref] [PubMed]
- Cushing MM, Asmis LM, Harris RM, et al. Efficacy of a new pathogen-reduced cryoprecipitate stored 5 days after thawing to correct dilutional coagulopathy in vitro. Transfusion 2019;59:1818-26. [Crossref] [PubMed]
- Kamyszek RW, Foster MW, Evans BA, et al. The effect of pathogen inactivation on cryoprecipitate: a functional and quantitative evaluation. Blood Transfus 2020;18:454-64. [PubMed]
Cite this article as: Godby RC, May JE, Lima JLO, Singh N, Marques MB. Congenital and acquired disorders of primary hemostasis. Ann Blood 2022;7:10.


