
A narrative review on progress and development of anti-CD36 antibody detection
Introduction
Immune-mediated thrombocytopenia occurs due to alloantibodies against platelet antigens, such as the ABO blood group antigens, HLA class I, and human platelet antigens (HPA). In recent years, more than 30 HPA have been identified (https://www.versiti.org/medical-professionals/precision-medicine-expertise/platelet-antigen-database/hpa-gene-database). Among them, alloantibodies against the HPA-1a formed by point mutation (Leu33Pro) on platelet glycoprotein (GP) IIIa (known as β3 integrin) are responsible for most cases of alloimmune thrombocytopenia in Caucasians (1). However, foetal and neonatal alloimmune thrombocytopenia (FNAIT) caused by anti-HPA-1a antibodies has not been well recognized in other populations. Interestingly, accumulating evidence indicates that immune-mediated thrombocytopenia caused by anti-CD36 antibodies is frequently found in Asian and African populations (2-5). CD36 isoantibodies (originally described as anti-Naka) was firstly reported in a Japanese patient who developed platelet-transfusion refractoriness (PTR) (6). According to the expression of CD36 on platelets and monocytes, two types of CD36 deficiency have been identified. Through blood transfusions or during pregnancy, type I deficient, but not type II, individuals may be at risk of developing anti-CD36 isoantibodies, leading to serious immune-mediated thrombocytopenia, such as FNAIT, PTR, and post-transfusion purpura (PTP) (4,7-10).
In recent years, the role of anti-CD36 antibodies in the pathophysiology of immune-mediated thrombocytopenia has been attracting much attention in Asia and across the world. In this review article, all currently available methods for the detection of anti-CD36 antibodies will be discussed. We present the following article in accordance with the Narrative Review reporting checklist (available at https://dx.doi.org/10.21037/aob-21-48).
Methods for anti-CD36 antibody testing
Table 1 shows the list of different methods applicable for the analysis of anti-CD36 antibodies in PTR and FNAIT cases (3,4,6,8,9,11-22). The advantages and disadvantages of these different assays are illustrated in Table 2.
Table 1
| Subjects | Age (years)/sex | Population | Immune-mediated thrombocytopenia | MAIPA results (Pos or Neg) (clone name of mAbs) | PakPlus, PSIFT, MPHA, MACE, PAKLx, PABA, ACA | References |
|---|---|---|---|---|---|---|
| Patient #1 | 36/F | Japanese | PTR | n.t | PSIFT | Ikeda et al., 1989 (6) |
| Patient #2 | 19/M | Japanese | PTR | n.t | MPHA | Fujino et al., 2001 (9) |
| Patient #3 | 36/M | ? | PTR | Neg (FA6-152; 1A7 and 131.4) | PakPlus | Flesch et al., 2008 (11) |
| Patient #4 | 5/F | Lebanese | PTR | Weak Pos (FA6-152) | PakPlus, MACE | Saw et al., 2010 (12) |
| Patient #5 | 70/M | Chinese | PTR | Neg (FA6-152) | PakPlus, MACE | Saw et al., 2010 (12) |
| Patient #6 | 7/M | Chinese | PTR | n.t | PakPlus | Yin et al., 2011 (13) |
| Patient #7 | 22/M | Chinese | PTR | Pos (FA6-152) | PakPlus, PSIFT | Xu et al., 2013 (3) |
| Patient #8 | 35/F | Chinese | PTR | Pos (FA6-152) | PakPlus, | Xia et al.,2014 (14) |
| Patient #9 | 26/F | Chinese | PTR | Pos (FA6-152) | PSIFT | Wu et al., 2014 (15) |
| Patient #10 | 21/F | Chinese | PTR | Pos (FA6-152) | PSIFT | Wu et al., 2014 (15) |
| Patient #11 | 63/F | Chinese | PTR | Pos (FA6-152) | PSIFT | Wu et al., 2014 (15) |
| Patient #12 | 21/M | Chaldean | PTR | n.t | PABA | Khatri et al., 2019 (16) |
| Patient #13 | 66/F | African-American | PTR | n.t | PakPlus, PABA | Schmidt et al., 2020 (17) |
| Patient #14 | ?/F | Thai | FNAIT | n.t | MPHA | Kankirawatana et al., 2001 (8) |
| Patient #15 | 37/F | Nigerian | FNAIT | n.t | PakPlus, MACE | Curtis et al., 2002 (4) |
| Patient #16 | 32/F | Italian | FNAIT | n.t | PakPlus, MACE | Curtis et al., 2002 (4) |
| Patient #17 | 28/F | African-American | FNAIT | n.t | PakPlus, MACE | Curtis et al., 2002 (4) |
| Patient #18 | 21/F | African-American | FNAIT | n.t | PakPlus, MACE | Curtis et al., 2002 (4) |
| Patient #19 | 36/F | Japanese | FNAIT | n.t | PSIFT | Okajima et al., 2006 (18) |
| Patient #20 | ?/F | Japanese | FNAIT | n.t | MPHA, PakPlus | Taketani et al., 2008 (19) |
| Patient #21 | 30/F | Chinese | FNAIT | Neg (FA6-152) | PakPlus, PSIFT, ACA | Xu et al., 2013 (3) |
| Patient #22 | 35/F | Chinese | FNAIT | Neg (FA6-152) | PakPlus, ACA | Xu et al., 2018 (20) |
| Patient #23 | 26/F | Taiwanese | FNAIT | Neg (FA6-152) | PSIFT, ACA | Lin et al., 2018 (21) |
| Patient #24 | 41/F | African | FNAIT | Neg (FA6-152; 10.5 and TR9) | PAKLx | Bertrand et al., 2019 (22) |
?, unknown; n.t, not tested; F, female; M, male; Pos, positive; Neg, negative. The ACA was also used to detect the anti-CD36 antibodies in patients #21–23. PTR, platelet transfusion refractoriness; FNAIT, foetal and neonatal alloimmune thrombocytopenia; PakPlus, the commercial solid-phase assay; MAIPA, monoclonal antibody-specific immobilization of platelet antigens; PSIFT, platelet suspension immunofluorescence test; MPHA, mixed passive hemagglutination assay; MACE, modified antigen capture ELISA; PAKLx, Luminex bead-based platelet antibody detection assay; PABA, platelet antibody bead array; ACA, monoclonal antibody-independent antigen capture assay.
Table 2
| Assay | Principle | Advantage | Disadvantage |
|---|---|---|---|
| PSIFT | Binding assays using intact platelets | Simple practicality, fast | Low specificity and sensitivity, requires a FACS instrument |
| MPHA | Simple practicality, low cost | Low specificity and sensitivity, anti-HLA antibodies interfere the detection of HPA antibodies | |
| MAIPA | Binding assays with immobilized platelet glycoproteins | High specificity, high sensitivity | Time-consuming, complicated procedure, false-negative results often occur |
| MACE | High specificity | High cost, false-negative results often occur | |
| PakPlus | High specificity, rapidity, convenient | High cost, not all platelet antibodies could be tested | |
| HP-IPA | Binding assays with CD36 transfected cell lines | High sensitivity | High cost, complicated procedure, transfected cell lines are required |
| ACA | High sensitivity | High cost, complicated procedure, transfected cell lines are required | |
| PAKLx | Simultaneous binding assays using fluorescent bead-coated antigens | High sensitivity, high throughput, rapidity, simplicity | High cost, requires a Luminex instrument and not all platelet antibodies could be tested |
PakPlus, the commercial solid-phase assay; MAIPA, monoclonal antibody-specific immobilization of platelet antigens; PSIFT, platelet suspension immunofluorescence test; MPHA, mixed passive hemagglutination assay; MACE, modified antigen capture ELISA; PAKLx, Luminex bead-based platelet antibody detection assay; ACA, monoclonal antibody-independent antigen capture assay; HP-IPA, HP cell-based mAb-independent immobilization of platelet antigens assay.
Binding assays with intact platelets
In the 1970s, von dem Borne et al. developed a simple platelet suspension immunofluorescence test (PSIFT) to detect platelet antibodies using paraformaldehyde-fixed platelets by fluorescence microscopy (23). Although PSIFT is sensitive, this method is difficult to standardize and does not provide an objective quantification, and therefore, analysis by flow cytometry is preferable (Figure 1A) (6). In Japan, a mixed passive hemagglutination assay (MPHA) using sheep red cells (MPHA) or magnetic beads (M-MPHA) coated with anti-human IgG as an indicator was established to detect platelet antibodies (Figure 1B) (24,25). By this approach, all antibodies reacting with antigens expressed on the platelet surface are detected. Consequently, identifying platelet-specific antibodies (anti-HPA) in a serum sample containing other platelet reactive antibodies (such as anti-HLA) is difficult. To avoid this problem, Nordhagen et al. denatured HLA antigenic determinants to produce HLAnull platelets by treatment with chloroquine (26).
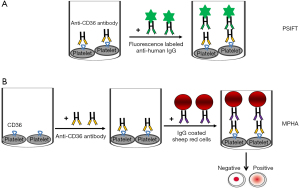
This approach, however, does not only cause reduction of HLA class I reactivity, but also diminished the binding of platelet specific antibodies (27). Nevertheless, due to simple practicality, flow cytometry and MPHA methods are widely used by many laboratories to identify platelet antibodies, including anti-CD36 antibodies (Table 2). In Table 1, 5 of 13 cases of PTR and 3 of 11 cases of FNAIT caused by anti-CD36 antibodies are tested by flow cytometry (3,6,15,18,21). MPHA was only used to test for anti-CD36 antibodies in one PTR case and two FNAIT cases reported in Japan (8,9,19). However, the use of control cells that do not express the antigen (CD36 negative) is mandatory to draw a final statement about the presence of anti-CD36 isoantibodies. Therefore, fresh platelets from CD36-deficient individuals are required to evaluate anti-CD36 antibodies, which are not easily available to many laboratories.
Binding assays with immobilized platelet glycoproteins
To avoid the interference of anti-HLA antibodies and to define the specificities of anti-HPA antibodies in a serum sample, a glycoprotein-specific immunoassay using monoclonal antibodies (mAbs) as capture antibodies was developed. The first generation of such assay was based on the use of platelet lysates. Platelet glycoproteins in platelet lysate were captured by mAbs, and antibodies from the serum sample bound to the immobilized glycoprotein were subsequently detected with radiolabelled secondary antibodies (28,29). Based on the disadvantages of this assay (radioactivity, high background, and low sensitivity), a second generation of the glycoprotein-specific immunoassay, known as MAIPA, was developed. In contrast to the first-generation assay, whole platelets were used. Platelets were first incubated with the serum sample and mAbs and then lysed. A triple molecular complex consisting of mAb-antigen-human antibodies was captured by immobilized anti-mouse IgG. Finally, bound human antibodies were detected by the use of enzyme-labeled secondary antibodies (Figure 2A) (30). This approach is currently considered the gold standard for the characterization of platelet antibodies (30,31). In addition, a modified antigen capture ELISA (MACE) was established to prevent non-specific reactions. In this method, human antibodies bound platelets were lysed and then added into the microtiter wells coated directly with mAbs against platelet glycoproteins (Figure 2B) (25). However, high sensitivity and specificity of antibody detection could be achieved by both approaches.
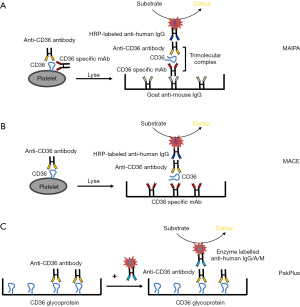
Twelve of the reported 24 PTR and FNAIT cases caused by anti-CD36 antibodies were evaluated by MAIPA using reference mAbs FA6-152 against CD36 as the capture antibody (3,11,12,14,15,20-22). MACE was used in six cases (Table 1) (5,12). Although both assays are frequently used, false-negative results often occur due to competitive inhibition between capture mouse mAbs and human anti-CD36 antibodies (32). In this cohort, MAIPA identified anti-CD36 antibodies in sera in only 6/12 cases (50.00%) (Table 1). In patient #5, negative results were found both in MAIPA and MACE using mAbs FA6-152 and MBC131.4 as capture antibodies, respectively (12). This observation indicates that the selection of mAbs against CD36 recognizing different epitopes that are distinct from human antibodies is important to minimize false negative result. Although this assay is time-consuming, this methodological approach has several advantages, allowing the quantification of antibodies, direct comparison with other platelet antibodies, and does not need CD36 negative platelets. More importantly, this assay allows cross-match analysis between maternal serum and paternal platelets representing antigen-antibody reaction in FNAIT (Table 2).
To simplify the detection of anti-CD36 antibodies, a commercialized ELISA (GTI PakPlus) was developed. This assay is based on the use of immobilized purified platelet glycoprotein CD36 and seems to be specific for identifying anti-CD36 antibodies (Figure 2C). As shown in Table 1, seven cases of FNAIT and seven cases of PTR caused by anti-CD36 could be identified by PakPlus (3,5,11-14,17,19,20). Although this assay is rapid and convenient, the GTI PakPlus Kit may fail to detect or may incorrectly identify clinically significant anti-HPA antibodies (Table 2) (33).
Binding assays with CD36 transfected cell lines
To overcome the shortage of low-frequency HPA typed or deficient platelets, a panel of different cell lines stably expressing platelet antigens on the cell surface have been established as an alternative source of phenotype platelets to detect platelet-specific alloantibodies in human serum (34-36). For the detection of anti-CD36 antibodies by flow cytometry, a stably transfected K562 cell line expressing CD36 antigen was introduced to detect anti-CD36 antibodies (37). Compared to other cell lines, K562 cells fail to express the HLA class I antigen; consequently, the presence of anti-HLA antibodies cannot influence this assay. To broaden the use of this K562 cell line expression CD36 (HP-CD36), a monoclonal antibody-independent immobilization of platelet antigens (HP-IPA) assay was developed (38). In this assay, HP-CD36 cells were first reacted with test serum and were then incubated with peroxidase-conjugated mouse anti-human IgG. After solubilization, the lysates in the supernatant were applied onto the microtiter wells pre-coated with goat anti-mouse IgG (Figure 3A).
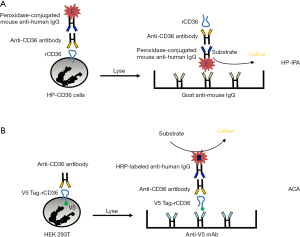
In our laboratory, another monoclonal antibody-independent antigen capture assay (ACA) was established (21). In this approach, recombinant CD36 harbouring a V5-peptide (GKPIPNPLLGLDST) was expressed on the surface of mammalian HEK293 cells. After incubating these cells with human sera, the bound anti-CD36 antibodies were detected in the cell lysates after immobilization of the CD36 antigen on a microtiter wells pre-coated with anti-V5 antibodies (Figure 3B). This method overcomes the abovementioned problem of false-negative reactions caused by competitive inhibition (Table 2). Notably, in patient #23, a negative result was obtained when MAIPA was performed with platelets using mAb FA6-152, but a positive result was obtained by ACA (21). Similar results were obtained with other serum samples (patients #21 and #22; Table 1).
Simultaneous binding assays using fluorescent bead-coated antigens
Recently, different approaches have been developed for high-throughput screening of platelet antibodies based on different fluorescence-labeled beads coated with platelet antigen, such as the simultaneous analysis of specific platelet antibodies (SASPA), immunocomplex capture fluorescence analysis (ICFA), and platelet antibody bead array (PABA) (39-41). In comparison to previous assays, all these methods allow the simultaneous analysis of different platelet antibody specificities. For example, by PABA, four serum samples containing anti-CD36 antibodies were correctly identified (41). As shown in Table 1, anti-CD36 antibodies in serum sample #12 could be detected by this method as well (16).
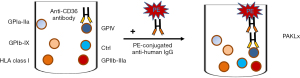
Meanwhile, a commercial Luminex bead-based platelet antibody detection method, PAKLx kit, is available. These fluorescence-labeled beads as targets are immobilized with platelet lysate-derived glycoproteins to capture and identify antibodies against HPA, HLA Class I or CD36 (Figure 4). Compared to the classical MAIPA, the PAKLx assay has several advantages (rapidity, simplicity, and platelets and mAbs are not needed) (Table 2). Interestingly, anti-CD36 antibodies could be identified in serum sample #24 (Table 1) by PAKLx, but not by MAIPA (22). However, the stability and heterogeneity of isolated antigens have to be taken into consideration. Accordingly, labile antigens, such as HPA-3 and HPA-15, cannot be reliably detected by this assay (42,43).
Real-time antibody binding assay
All antibody binding assays described above include several washing steps and only allow end-point readout. Therefore, low-avidity antibodies may be overlooked. To overcome this problem, a real-time antigen-antibody binding analysis by surface plasmon resonance technology (SPR) has been developed (44-46). By using a purified HPA-1a antigen, Bakchoul et al. demonstrated the existence of low-avidity anti-HPA-1a alloantibodies, which were undetectable by MAIPA (45). This observation was confirmed by another study (46). Similar to PakLx, the isolation of native purified antigens is a difficult hurdle for this approach.
Conclusions
Several methods have been developed to detect anti-CD36 antibodies. However, for routine testing, every assay still has some limitations. Currently, a combination of these methods is necessary to ensure the detection of anti-CD36 antibodies. Nevertheless, further improvement of MAIPA and MACE should be in the foreground. This approach currently allows cross-match analysis between maternal antibody and foetal antigen that reflects antigen-antibody reaction occurring in FNAIT. In addition, only this approach permits the identification of new platelet antigens residing on CD36.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding: This work was supported in part by The Key Medical Disciplines and Specialties Program of Guangzhou (No. 2021-2023).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Brian R. Curtis) for the series “Thrombocytopenia Due to Immunization Against CD36” published in Annals of Blood. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/aob-21-48
Peer Review File: Available at https://dx.doi.org/10.21037/aob-21-48
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/aob-21-48). The series “Thrombocytopenia Due to Immunization Against CD36” was commissioned by the editorial office without any funding or sponsorship. SS serves as an unpaid editorial board member of Annals of Blood. XX serves as an unpaid Section Editor of Annals of Blood. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mueller-Eckhardt C, Kiefel V, Grubert A, et al. 348 cases of suspected neonatal alloimmune thrombocytopenia. Lancet 1989;1:363-6. [Crossref] [PubMed]
- Wu G, Zhou Y, Li L, et al. Platelet Immunology in China: Research and Clinical Applications. Transfus Med Rev 2017;31:118-25. [Crossref] [PubMed]
- Xu X, Ye X, Xia W, et al. Studies on CD36 deficiency in South China: Two cases demonstrating the clinical impact of anti-CD36 antibodies. Thromb Haemost 2013;110:1199-206. [Crossref] [PubMed]
- Curtis BR, Ali S, Glazier AM, et al. Isoimmunization against CD36 (glycoprotein IV): description of four cases of neonatal isoimmune thrombocytopenia and brief review of the literature. Transfusion 2002;42:1173-9. [Crossref] [PubMed]
- Lee K, Godeau B, Fromont P, et al. CD36 deficiency is frequent and can cause platelet immunization in Africans. Transfusion 1999;39:873-9. [Crossref] [PubMed]
- Ikeda H, Mitani T, Ohnuma M, et al. A new platelet-specific antigen, Naka, involved in the refractoriness of HLA-matched platelet transfusion. Vox Sang 1989;57:213-7. [Crossref] [PubMed]
- Yamamoto N, Akamatsu N, Sakuraba H, et al. Platelet glycoprotein IV (CD36) deficiency is associated with the absence (type I) or the presence (type II) of glycoprotein IV on monocytes. Blood 1994;83:392-7. [Crossref] [PubMed]
- Kankirawatana S, Kupatawintu P, Juji T, et al. Neonatal alloimmune thrombocytopenia due to anti-Nak(a). Transfusion 2001;41:375-7. [Crossref] [PubMed]
- Fujino H, Ohta K, Taniue J, et al. Primary refractoriness to platelet transfusion caused by Nak(a) antibody alone. Vox Sang 2001;81:42-4. [Crossref] [PubMed]
- Bierling P, Godeau B, Fromont P, et al. Posttransfusion purpura-like syndrome associated with CD36 (Naka) isoimmunization. Transfusion 1995;35:777-82. [Crossref] [PubMed]
- Flesch B, Miller J, Repp R, et al. Successful autologous hematopoietic progenitor cell transplantation in a patient with an isoantibody against CD36 (glycoprotein IV, Naka). Bone Marrow Transplant 2008;42:489-91. [Crossref] [PubMed]
- Saw CL, Szykoluk H, Curtis BR, et al. Two cases of platelet transfusion refractoriness associated with anti-CD36. Transfusion 2010;50:2638-42. [Crossref] [PubMed]
- Yin XL, Shen WD, Chen YS, et al. Refractory platelet transfusion in a patient with CD36 deficiency due to pseudothrombocytopenia. Platelets 2011;22:237-40. [Crossref] [PubMed]
- Xia W, Ye X, Xu X, et al. Two cases of platelet transfusion refractoriness and one case of possible FNAIT caused by antibodies against CD36 in China. Transfus Med 2014;24:254-6. [Crossref] [PubMed]
- Wu GG. Detection of clinically relevant platelet antibodies in the Asian population. ISBT Sci Ser 2014;9:112-7. [Crossref]
- Khatri SS, Curtis BR, Yamada C. A case of platelet transfusion refractoriness due to anti-CD36 with a successful treatment outcome. Immunohematology 2019;35:139-44. [Crossref] [PubMed]
- Schmidt AE, Sahai T, Refaai MA, et al. Severe Platelet Transfusion Refractoriness in Association with Antibodies Against CD36. Lab Med 2020;51:540-4. [Crossref] [PubMed]
- Okajima S, Cho K, Chiba H, et al. Two sibling cases of hydrops fetalis due to alloimmune anti-CD36 (Nak a) antibody. Thromb Haemost 2006;95:267-71. [Crossref] [PubMed]
- Taketani T, Ito K, Mishima S, et al. Neonatal isoimmune thrombocytopenia caused by type I CD36 deficiency having novel splicing isoforms of the CD36 gene. Eur J Haematol 2008;81:70-4. [Crossref] [PubMed]
- Xu X, Li L, Xia W, et al. Successful management of a hydropic fetus with severe anemia and thrombocytopenia caused by anti-CD36 antibody. Int J Hematol 2018;107:251-6. [Crossref] [PubMed]
- Lin M, Xu X, Lee HL, et al. Fetal/neonatal alloimmune thrombocytopenia due to anti-CD36 antibodies: antibody evaluations by CD36-transfected cell lines. Transfusion 2018;58:189-95. [Crossref] [PubMed]
- Bertrand G, Renac V, Lefaix MC, et al. Neonatal intracranial hemorrhage with a dramatic outcome due to maternal anti CD36 antibodies. Reports 2019;2:7. [Crossref]
- von dem Borne AE, Verheugt FW, Oosterhof F, et al. A simple immunofluorescence test for the detection of platelet antibodies. Br J Haematol 1978;39:195-207. [Crossref] [PubMed]
- Shibata Y, Juji T, Nishizawa Y, et al. Detection of platelet antibodies by a newly developed mixed agglutination with platelets. Vox Sang 1981;41:25-31. [Crossref] [PubMed]
- Matsuhashi M, Tsuno NH. Laboratory testing for the diagnosis of immune-mediated thrombocytopenia. Ann Blood 2018;3:41. [Crossref]
- Nordhagen R, Flaathen ST. Chloroquine removal of HLA antigens from platelets for the platelet immunofluorescence test. Vox Sang 1985;48:156-9. [Crossref] [PubMed]
- Langenscheidt F, Kiefel V, Santoso S, et al. Quantitation of platelet antigens after chloroquine treatment. Eur J Haematol 1989;42:186-92. [Crossref] [PubMed]
- Woods VL Jr, Oh EH, Mason D, et al. Autoantibodies against the platelet glycoprotein IIb/IIIa complex in patients with chronic ITP. Blood 1984;63:368-75. [Crossref] [PubMed]
- Woods VL Jr, Kurata Y, Montgomery RR, et al. Autoantibodies against platelet glycoprotein Ib in patients with chronic immune thrombocytopenic purpura. Blood 1984;64:156-60. [Crossref] [PubMed]
- Kiefel V, Santoso S, Weisheit M, et al. Monoclonal antibody--specific immobilization of platelet antigens (MAIPA): a new tool for the identification of platelet-reactive antibodies. Blood 1987;70:1722-6. [Crossref] [PubMed]
- Menitove JE, Pereira J, Hoffman R, et al. Cyclic thrombocytopenia of apparent autoimmune etiology. Blood 1989;73:1561-9. [Crossref] [PubMed]
- Morel-Kopp MC, Daviet L, McGregor J, et al. Drawbacks of the MAIPA technique in characterising human antiplatelet antibodies. Blood Coagul Fibrinolysis 1996;7:144-6. [Crossref] [PubMed]
- Lucas GF, Rogers SE. Evaluation of an enzyme-linked immunosorbent assay kit (GTI PakPlus(R)) for the detection of antibodies against human platelet antigens Transfus Med 1999;9:385-6. [Crossref] [PubMed]
- Hayashi T, Amakishi E, Inoue M, et al. Establishment of a cell line panel for the detection of antibodies against human platelet antigen 4b. Int J Hematol 2011;93:170-5. [Crossref] [PubMed]
- Hayashi T, Amakishi E, Matsuyama N, et al. Detection of anti-human platelet antibodies against integrin α2β1 using cell lines. Blood Transfus 2014;12:s273-80. [PubMed]
- Hayashi T, Amakishi E, Matsuyama N, et al. Detection of antibodies against human platelet antigens 15a and 15b by using a cell line panel. Br J Haematol 2010;151:402-4. [Crossref] [PubMed]
- Hayashi T, Yasui K, Matsuyama N, et al. Establishment of a novel method for detecting Nak antibodies by using a panel cell line. Transfusion 2009;49:390-2. [Crossref] [PubMed]
- Amakishi E, Hayashi T, Koh Y, et al. A new transfectant panel cell line-based MoAb-independent antigen capture assay system for detection of CD36 antibody. Vox Sang 2014;106:368-71. [Crossref] [PubMed]
- Nguyen XD, Dugrillon A, Beck C, et al. A novel method for simultaneous analysis of specific platelet antibodies: SASPA. Br J Haematol 2004;127:552-60. [Crossref] [PubMed]
- Fujiwara K, Shimano K, Tanaka H, et al. Application of bead array technology to simultaneous detection of human leucocyte antigen and human platelet antigen antibodies. Vox Sang 2009;96:244-51. [Crossref] [PubMed]
- Metzner K, Bauer J, Ponzi H, et al. Detection and identification of platelet antibodies using a sensitive multiplex assay system-platelet antibody bead array. Transfusion 2017;57:1724-33. [Crossref] [PubMed]
- Porcelijn L, Huiskes E, Comijs-van Osselen I, et al. A new bead-based human platelet antigen antibodies detection assay versus the monoclonal antibody immobilization of platelet antigens assay. Transfusion 2014;54:1486-92. [Crossref] [PubMed]
- Cooper N, Bein G, Heidinger K, et al. A bead-based assay in the work-up of suspected platelet alloimmunization. Transfusion 2016;56:115-8. [Crossref] [PubMed]
- Socher I, Andrei-Selmer C, Bein G, et al. Low-avidity HPA-1a alloantibodies in severe neonatal alloimmune thrombocytopenia are detectable with surface plasmon resonance technology. Transfusion 2009;49:943-52. [Crossref] [PubMed]
- Bakchoul T, Kubiak S, Krautwurst A, et al. Low-avidity anti-HPA-1a alloantibodies are capable of antigen-positive platelet destruction in the NOD/SCID mouse model of alloimmune thrombocytopenia. Transfusion 2011;51:2455-61. [Crossref] [PubMed]
- Peterson JA, Kanack A, Nayak D, et al. Prevalence and clinical significance of low-avidity HPA-1a antibodies in women exposed to HPA-1a during pregnancy. Transfusion 2013;53:1309-18. [Crossref] [PubMed]
Cite this article as: Xu X, Santoso S. A narrative review on progress and development of anti-CD36 antibody detection. Ann Blood 2021;6:35.


