
CD36 immunization causing platelet transfusion refractoriness: narrative review
Overview of platelet transfusion refractoriness (PTR)
PTR is commonly encountered in clinical practice (1). Most cases [over 80% (2)] are due to non-immune mediated mechanisms that increase the consumption of transfused platelets, while a smaller proportion is due to immune-mediated mechanisms, where transfused platelets are rapidly cleared from circulation. Alloimmunization most frequently involves class I human leukocyte antigen (HLA) antigens, but platelet antigens such as glycoprotein (GP) IV (CD36) can be implicated. With clinically significant bleeding present in over 1/3 of patients with immune-mediated platelet alloimmunization and a bleeding-related mortality rate of around 8% (3), identifying the specific antigen involved is crucial for providing effective transfusion support. From the perspective of blood product utilization and cost, up to 2/3 of all transfused platelet units are administered to patients with PTR and PTR is associated with longer hospitalization and higher cost per admission (4). This review aims to describe the clinical and laboratory approach to patients with PTR from CD36 immunization and provide tools for clinicians and laboratory specialists to care for patients with this rare and challenging presentation. We present the following article in accordance with the Narrative Review reporting checklist (available at https://dx.doi.org/10.21037/aob-21-36).
Establishing the diagnosis of PTR
Following the observation of a platelet transfusion yield lower than expected, systematic evaluation of post transfusion counts will confirm the diagnosis. Most studies use the corrected count increment (CCI) as a standardized measure, which adjusts for body surface area and dose of platelets administered (5). To distinguish non-immune vs. immune-mediated causes, a platelet count within 1 hour of transfusion is obtained. A CCI <5,000 or an absolute increase of under 10,000/µL immediately after transfusion (confirmed in duplicate) is suggestive of immune-mediated PTR, however concomitant non-immune mediated causes should always be investigated.
Suspecting CD36 immunization as the cause of PTR
Once immune-mediated PTR has been established, a work-up to determine the specific antigen(s) involved is initiated. The type of laboratory studies obtained, turnaround time of results and transfusion strategy pursued while awaiting results, vary across facilities and countries and depend greatly on availability of local resources and geographical distance to reference testing and blood banks.
The prevalence of platelet GP IV deficiency varies across ethnicities. In the Sub-Saharan African population, CD36 negativity is as high as 7.7% (6). Chinese (7-9) and African American (10) populations have a CD36 negativity prevalence between 2.2% and 2.4%, followed by the Japanese population at 1% (11) and is lowest for Western Europeans where it approaches 0% (6). The patient’s ancestry can increase the pre-test probability of PTR being from antibodies against CD36.
Platelet crossmatching is used in some centers to select the most compatible platelet units while awaiting results from antibody screening assays. Considering the very low frequency of CD36 negative individuals in most populations, a CD36 antibody is expected to lead to incompatibility of most (if not all) platelet units crossmatched. An incompatible crossmatch with multiple units will not be exclusive to CD36 immunization as it can be seen with class I HLA antibodies and antibodies to other common platelet antigens.
Since class I HLA antibodies are the cause of the majority of immune-mediated PTR, testing for class I HLA antibodies and finding HLA-matched platelets in the event an HLA antibody is present, can greatly improve platelet survival. In patients without class I HLA antibodies or those that continue to have a low platelet transfusion yield with HLA-matched platelet transfusions, investigating platelet antigen related refractoriness is indicated.
Confirming CD36 immunization
Antibody screening assays, including the PakPlus (LIFECODES, Waukesha, WI; Immucor GTI Diagnostics, Inc.) detect class I HLA, CD36, and GP IIb/IIIa, GP Ia/IIa and GP Ib/IX antibodies. Antigen capture assays, including modified antigen capture ELISA (MACE) and platelet antibody bead assay (PABA) (12) will delineate specificity of platelet antibodies and will not have interference from non-HPA antibodies (Figure 1). In rare instances, the CD36 mouse anti-human monoclonal antibody can block human antibody binding by competing for the same nearby epitope which prevents proper antibody detection and can lead to false negative results (13). Using a second CD36 monoclonal antibody can ameliorate this issue and increase the sensitivity of the assay.
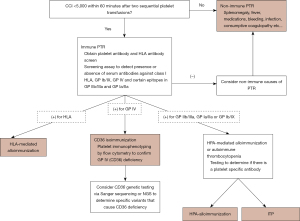
Once CD36 reactivity (antibody) is detected, immunophenotyping of the patient’s platelets and/or monocytes by flow cytometry should be considered. There are two types of CD36 deficiency, type I where both platelets and monocytes lack CD36 and type II in which only platelets do not express CD36. Type II deficiency is more common (7). It is widely accepted that only type I deficient individuals can produce antibodies, and hence it may only be necessary to test platelets (as absence of CD36 on monocytes would be assumed). However, patients with type II CD36 deficiency and detectable antibody have been reported in the literature (6). It is worth noting that the detection of CD36 antibodies is performed at reference laboratories and flow cytometry immunophenotyping is not widely available. Genotyping of the CD36 gene is expected to reveal either homozygous or compound heterozygous variants and support the diagnosis of CD36 deficiency.
A large number of population estimates of CD36 deficiency have been established by phenotypic studies on platelets of blood donors and do not distinguish between the two types of deficiency (1). Studies that have reported phenotyping of both platelets and monocytes using flow cytometry demonstrate that while PTR is expected to affect almost exclusively those with type I deficiency, individuals with type II deficiency are suitable donors for patients with PTR caused by CD36 antibodies.
The identification of clinically relevant anti-CD36 antibodies is best accomplished in the context of immune-mediated PTR where in vivo data supports that the antibody is not only present but actively participating in clearance of CD36 positive transfused platelets.
In some clinical contexts, platelet antibody screens are obtained prior to intensive chemotherapy (such as that required for allogeneic stem cell transplantation) and CD36 antibodies are identified, yet the individuals are subsequently found to have adequate response to transfused platelets (14).
Transfusion support for the patient with PTR from CD36 antibodies
Platelet transfusions from CD36 negative donors are the main modality of support for patients with PTR from CD36 immunization. These platelets units are difficult to obtain, especially in geographical regions where the donor population has a low prevalence of CD36 deficiency. Screening family members to identify those who may also be CD36 deficient and suitable to serve as platelet donors should be considered (Figure 2).

While globalization has facilitated exchange of therapeutic products, geographical distance from a large/reference blood bank can significantly limit access to CD36 negative platelet products. Patients in Canada have successfully been transfused with CD36 negative platelets from the United States (20), while a patient in Germany was unable to receive platelet units from Japan, as the 18- to 24-hour travel time would have compromised viability of the product (25). For the patient in Germany, autologous hematopoietic stem cell transplant conditioning was postponed until four CD36 negative family members were identified and traveled to Germany to provide directed platelet donations.
Even when CD36 negative platelet units are available, the presence of concomitant class I HLA antibodies or non-immune platelet refractoriness may lead to continued lack of response to platelet transfusion. Obtaining ABO compatible, HLA-matched and CD36 negative platelets becomes extremely difficult, if not impossible (26).
Clinical characteristics of patients with PTR from CD36 antibodies
Cases of PTR from CD36 immunization have been described in patients of various ethnicities and affecting males and females alike. Most have malignancy, in particular a hematologic malignancy, as the underlying cause of their need for platelet transfusions and many (but not all) had been previously transfused and most responded to CD36 negative platelets. Figure 2 summarizes the clinical data of 13 cases of PTR from CD36 immunization reported in the literature. While more cases have been reported, only those with detailed clinical information are presented.
Other therapies
When CD36 negative platelets are not available or patients are immunized against multiple antigens, adjuvant treatments can be considered. Strategies to increase transfusion yield in the context of anti-CD36 immunization leading to PTR such as polyvalent immunoglobulin (IVIG) to increase the half-life of transfused platelets and B-lymphocyte selective immunosuppression with rituximab have been described (26). The use of additional immunosuppressive agents to specifically address PTR is more likely to be utilized in patients who are not already undergoing treatment regimens that suppress the immune system. De-escalation of treatment intensity in the case of patients with hematologic malignancies has also been described, with the goal of allowing endogenous platelet count renewal in a shorter timeframe, albeit compromising the long-term outcomes in terms on control of the underlying malignancy (27).
Future directions
Despite wider access to testing and antigen negative platelet units, the management of patients with PTR from CD36 antibodies remains challenging. Continuing efforts to assure that the population of blood donors adequately reflects the racial makeup of the population it serves is key to the bility to adequately meet the needs of patients that need transfused products.
Advances in genome editing technology allowing for the engineering of antigen negative/universal platelets using technologies such as CRISPR/Cas9 promises to change the way we support patients who require special products (28).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Brian R. Curtis) for the series “Thrombocytopenia Due to Immunization Against CD36” published in Annals of Blood. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/aob-21-36
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/aob-21-36). The series “Thrombocytopenia Due to Immunization Against CD36” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Legler TJ, Fischer I, Dittmann J, et al. Frequency and causes of refractoriness in multiply transfused patients. Ann Hematol 1997;74:185-9. [Crossref] [PubMed]
- Doughty HA, Murphy MF, Metcalfe P, et al. Relative importance of immune and non-immune causes of platelet refractoriness. Vox Sang 1994;66:200-5. [Crossref] [PubMed]
- Goswamy RV, Wilson NR, Tannenbaum DJ, et al. Practice patterns and clinical outcomes of platelet alloimmunization in a comprehensive cancer center. Transfus Apher Sci 2021;60:103096. [Crossref] [PubMed]
- Meehan KR, Matias CO, Rathore SS, et al. Platelet transfusions: utilization and associated costs in a tertiary care hospital. Am J Hematol 2000;64:251-6. [Crossref] [PubMed]
- Trial to Reduce Alloimmunization to Platelets Study Group. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med 1997;337:1861-9. [Crossref] [PubMed]
- Lee K, Godeau B, Fromont P, et al. CD36 deficiency is frequent and can cause platelet immunization in Africans. Transfusion 1999;39:873-9. [Crossref] [PubMed]
- Li R, Qiao Z, Ling B, et al. Incidence and molecular basis of CD36 deficiency in Shanghai population. Transfusion 2015;55:666-73. [Crossref] [PubMed]
- Ma C, Wang J, Yang L, et al. A single-center investigational study of CD36 antigen deficiency and platelet alloantibody distribution in different populations in Northern China as well as platelet alloantibodies effect on pregnancy. Clin Chim Acta 2019;498:68-75. [Crossref] [PubMed]
- Xu X, Ye X, Xia W, et al. Studies on CD36 deficiency in South China: Two cases demonstrating the clinical impact of anti-CD36 antibodies. Thromb Haemost 2013;110:1199-206. [Crossref] [PubMed]
- Curtis BR, Aster RH. Incidence of the Nak(a)-negative platelet phenotype in African Americans is similar to that of Asians. Transfusion 1996;36:331-4. [Crossref] [PubMed]
- Yanai H, Chiba H, Fujiwara H, et al. Phenotype-genotype correlation in CD36 deficiency types I and II. Thromb Haemost 2000;84:436-41. [Crossref] [PubMed]
- Metzner K, Bauer J, Ponzi H, et al. Detection and identification of platelet antibodies using a sensitive multiplex assay system-platelet antibody bead array. Transfusion 2017;57:1724-33. [Crossref] [PubMed]
- Wu GG, Kaplan C, Curtis BR, et al. Report on the 14th International Society of Blood Transfusion Platelet Immunology Workshop. Vox Sang 2010;99:375-81. [Crossref] [PubMed]
- Culler EE, Hillyer CD, Haight AE, et al. CD36 immunization in a patient undergoing hematopoietic stem cell transplantation. Pediatr Blood Cancer 2008;50:660-2. [Crossref] [PubMed]
- Ikeda H, Mitani T, Ohnuma M, et al. A new platelet-specific antigen, Naka, involved in the refractoriness of HLA-matched platelet transfusion. Vox Sang 1989;57:213-7. [Crossref] [PubMed]
- Fujino H, Ohta K, Taniue J, et al. Primary refractoriness to platelet transfusion caused by Nak(a) antibody alone. Vox Sang 2001;81:42-4. [Crossref] [PubMed]
- Ogata T, Ohto H, Yasuda H, et al. CD36 (Naka) sensitization with platelet-transfusion refractoriness in a liver transplant recipient. Transplantation 2005;79:620. [Crossref] [PubMed]
- Flesch B, Miller J, Repp R, et al. Successful autologous hematopoietic progenitor cell transplantation in a patient with an isoantibody against CD36 (glycoprotein IV, Naka). Bone Marrow Transplant 2008;42:489-91. [Crossref] [PubMed]
- Broderick WR, Toor AA, Curtis BR, et al. Refractory thrombocytopenia due to allo-immune anti-CD36 complicating unrelated donor bone marrow transplant in a CD36-negative recipient. Blood 2007;110:4951. [Crossref]
- Saw CL, Szykoluk H, Curtis BR, et al. Two cases of platelet transfusion refractoriness associated with anti-CD36. Transfusion 2010;50:2638-42. [Crossref] [PubMed]
- Xu X, Ye X, Xia W, et al. Studies on CD36 deficiency in South China: Two cases demonstrating the clinical impact of anti-CD36 antibodies. Thromb Haemost 2013;110:1199-206. [Crossref] [PubMed]
- Xia W, Ye X, Xu X, et al. Two cases of platelet transfusion refractoriness and one case of possible FNAIT caused by antibodies against CD36 in China. Transfus Med 2014;24:254-6. [Crossref] [PubMed]
- Khatri SS, Curtis BR, Yamada C. A case of platelet transfusion refractoriness due to anti-CD36 with a successful treatment outcome. Immunohematology 2019;35:139-44. [Crossref] [PubMed]
- Schmidt AE, Sahai T, Refaai MA, et al. Severe Platelet Transfusion Refractoriness in Association with Antibodies Against CD36. Lab Med 2020;51:540-4. [Crossref] [PubMed]
- Flesch B, Miller J, Repp R, et al. Successful autologous hematopoietic progenitor cell transplantation in a patient with an isoantibody against CD36 (glycoprotein IV, Naka). Bone Marrow Transplant 2008;42:489-91. [Crossref] [PubMed]
- Khatri SS, Curtis BR, Yamada C. A case of platelet transfusion refractoriness due to anti-CD36 with a successful treatment outcome. Immunohematology 2019;35:139-44. [Crossref] [PubMed]
- Schmidt AE, Sahai T, Refaai MA, et al. Severe Platelet Transfusion Refractoriness in Association with Antibodies Against CD36. Lab Med 2020;51:540-4. [Crossref] [PubMed]
- Lawrence M, Mueller A, Ghevaert C. Using genome editing to engineer universal platelets. Emerg Top Life Sci 2019;3:301-11. [Crossref] [PubMed]
Cite this article as: Sullivan MJ, Perez Botero J. CD36 immunization causing platelet transfusion refractoriness: narrative review. Ann Blood 2021;6:37.


