
Transfusion of a platelet pool contaminated with exotoxin-producing Staphylococcus aureus: a case report
Introduction
Bacterial contamination of blood components is the leading cause of transfusion-transmitted septic infections (1). The transmission of bacterial infectious agents through transfusable blood products can cause post-transfusion reactions, posing severe risk of morbidity and mortality to recipient patients (1-4). The storage conditions of platelet concentrates (PCs) at 20–24 ℃ under agitation, in gas-permeable bags for up to seven days, renders bacteria with an amenable environment for proliferation (1,5). Canadian Blood Services and other blood centres worldwide have implemented multifaceted safety procedures to mitigate the risk of supplying and transfusing bacterially-contaminated PCs. These include donor screening questionnaire, disinfection of donor’s skin, diversion of the first aliquot (30–40 mL) of donated blood, and PC screening for the presence of bacteria or PC treatment with pathogen reduction technologies (1,5). Despite these precautions, reports of septic transfusion cases from infused PCs still occur due to bacterial contamination (2-4). Canada like most nations, has documented fatal transfusion cases due to bacterially-contaminated PCs (6). Furthermore, from August 2017 to December 2019, approximately 1 in 1,000 false-negative screening results were obtained during testing of outdated PCs at Canadian Blood Services (6). Evidence collected over a decade show that among aerobic bacterial contaminants isolated, staphylococcal species are predominant, with Staphylococcus aureus emerging as a frequent PC contaminant causing transfusion septic cases in Canada, Ireland, the US, and the UK (1,7-11).
S. aureus possess the tendency to escape detection during routine PC screening with automated culture methods due to slow growth and surface aggregates (biofilms) formation that adhere to platelets and platelet storage containers (7,12). In 2017, Canadian Blood Services reported a false negative septic transfusion event involving a platelet pool that had visible platelet aggregation and was contaminated with S. aureus (7). Héma-Québec (11), the National Health Service Blood and Transplant (NHSBT) (10), and the Centre for Disease Control (9) have documented similar cases of transfusion reactions involving PCs contaminated with S. aureus, which was missed during routine PC screening using automated culture systems. Symptoms observed in recipients of PCs contaminated with S. aureus resemble those cases involving Gram negative organisms. This is likely due to the presence of exotoxins that trigger septic shock (2,7). Superantigen exotoxins produced by S. aureus are important virulence factors of this organism and include the toxic shock syndrome toxin-1 (TSST-1), staphylococcal enterotoxins (SE) types A to E, G to Q, and SE-like toxins types R to Y, in addition to superantigen-like (SSL) toxins (13). Most S. aureus isolates produce these toxins, which can be extremely detrimental to recipients when transmitted through PC infusion (2,7). The severity of the clinical outcome manifested by transfusion patients is dependent on the virulence of the S. aureus strain, the type and concentration of superantigen toxin(s) secreted into the infused PC, and the patients’ immune status (2,7). Symptoms and sequelae caused by these toxins range from mild like fever and/or emesis to severe such as toxic shock and even death. Superantigen concentrations of less than 0.1 pg/mL can cause fever, shock, and hypotension (14). This report describes a false-negative septic transfusion event of an elderly male patient that occurred at a Canadian hospital, after being infused with a 5-day old buffy coat platelet pool that was contaminated with S. aureus producing enterotoxin type G, which has not been reported before. We present the following case in accordance with the CARE reporting checklist (available at https://aob.amegroups.com/article/view/10.21037/aob-21-38/rc).
Case presentation
In September 2020, an elderly male patient suffering from acute promyelocytic leukemia (APL) was transfused with an irradiated 5-day old buffy coat platelet pool at a Canadian hospital as part of the treatment for coagulopathy likely related to APL. At approximately five minutes into the transfusion, his heart and respiratory rate increased and his blood pressure dropped from baseline (Table 1); the transfusion was immediately terminated. Forty minutes post-transfusion, the patient developed an onset of severe chest pain, shortness of breath, chills/rigors, nausea/vomiting, transient hypoxemia, and fever, with changes in his vital signs as shown in Table 1. The patient was initially admitted with febrile neutropenia but was afebrile some days prior to the reaction. The patient did not receive any medication prior to PC transfusion. Following the reaction, analgesics and nitroglycerine spray were administered. Once the patient’s chest pain was resolved, blood samples were taken for microbiological investigation and Tazocin was administered. A day after the septic reaction, the patient was afebrile and additional blood products were infused with no reported reactions. A computed tomography (CT) chest scan revealed patient improvement; Caspofungin and Cefazolin were added as supplementary treatment. PCs are screened for bacterial contamination at Canadian Blood Services with the automated BACT/ALERT culture system using a large volume delayed sampling algorithm as described by Ramirez-Arcos et al. (6). The BACT/ALERT testing result of the PC implicated in this case, after 7 days of bottle incubation, was negative. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Table 1
| Vital signs | Transfusion | ||
|---|---|---|---|
| Pre | Duringa | Postb | |
| Temperature (℃) | 37.1 | 37.1 | 39.1 |
| Heart rate | 87 | 89 | 123 |
| Respiration | 20 | 22 | 24 |
| Blood pressure | 137/69 | 107/56 | 140/69 |
| Oxygen saturation (%) | 98 | 88 | 94 |
a, patient experiences chest pain. Nitro spray is given, but it is ineffective. b, chest pain has resolved. Blood cultures were taken and tazocin started.
Clinical findings
Samples from peripheral blood, peripherally inserted central catheter (PICC) line, and residual infused PC unit were analysed at the hospital microbiology laboratory. The four donors of the implicated platelet pool had donated between 3 and 122 times before this donation and were not associated with previous adverse transfusion events. They also showed no signs of systematic illness at the time of donation. Patient’s blood and residual PC samples were cultured at the hospital and all were positive in less than 3.5 hours of incubation in the BACT/ALERT system. Gram staining of the positive cultures revealed Gram positive cocci, which were further speciated as S. aureus.
Antibiotic susceptibility testing revealed susceptibility of both S. aureus isolates (from the patient and PC sample) to cefazolin and cloxacillin, and resistance to clindamycin. The remaining transfused PC and associated four red blood cell (RBC) units were sent to the Canadian Blood Services Centre for Innovation Microbiology Laboratory (Micro Lab) for further testing with the BACT/ALERT system as per standard protocols (15). All four co-component RBC units tested negative for bacterial contamination; this result may be due to lack of S. aureus proliferation in RBC units resulting in sampling error or to self-sterilization in this blood component.
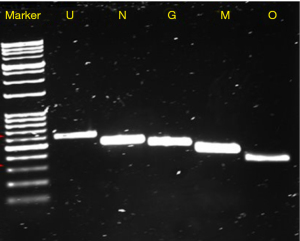
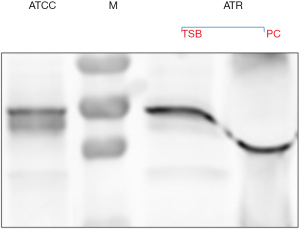
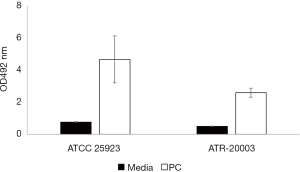
Quantification of S. aureus in the residual PC, performed in the Micro Lab, showed that the unit contained approximately 109 colony-forming units per millilitre (CFU/mL), a concentration that is clinically significant (16). The S. aureus strain isolated from the PC was assigned with the identification number ATR-20003 as per Canadian Blood Services standard procedures. Genomic DNA was extracted from this isolate and assayed for the presence of 21 superantigen enterotoxin genes by polymerase chain reaction (PCR) performed under the following optimized conditions: 30 s initial denaturation at 95 ℃; 30 cycles of denaturation (30 s at 95 ℃), annealing (1 min at 58 ℃), and extension (1 min at 68 ℃); a final extension at 68 ℃ for 5 mins (16). The primer sets that yielded amplicons are listed in Table 2. The results revealed that the genome of this new isolate encodes for five enterotoxins including SEs-G, M, N, O and SE-like U (Figure 1). The PCR amplicons were sequenced and the identity of the five genes encoding for these enterotoxins was confirmed. Importantly, enterotoxin SEG was detected by Western blotting in both, a culture supernatant of S. aureus ATR-20003 and a sample of the transfused PC (Figure 2). Since this isolate escaped detection during routine screening, its ability to form surface attached aggregates known as biofilms was assessed in PCs. A semi quantitative assay was performed as previously described (9) with a few modifications. Briefly, following the establishment of biofilms, the adherent cells were fixed with methanol (100% v/v) prior to staining with crystal violet. The results indicate that S. aureus ATR-20003 forms strong biofilms in PCs (Figure 3).
Table 2
| Gene | Primers | Oligonucleotide sequence (5' to 3') | Size of amplified product (bp) |
|---|---|---|---|
| seg | SEG-F | GAATGCTCAACCCGATCCTAA | 491 |
| SEG-R | GCCAGTGTCTTGCTTTGTAATC | ||
| sem | SEM-F | TTCGACAGTAACAGCTGAATTT | 440 |
| SEM-R | GCCCTGTTCCTGTATCAAATAA | ||
| sen | SEN-F | CGTGGCAATTAGACGAGTCA | 524 |
| SEN-R | AAACTCTGCTCCCACTGAAC | ||
| seo | SEO-F | GTCAAGTGTAGACCCTATTGC | 364 |
| SEO-R | ATCCTTATACACAGCTACTCCT | ||
| selu | SElU-F | GGCAATCCTAGACCAGAACAA | 627 |
| SElU-F | TTCACCAGATTCAGGCATCAT |
SE, staphylococcal enterotoxin.
Discussion
Clinical sequelae of infused bacterially-contaminated PCs are variable and may be acute or delayed. The severity of the transfusion clinical outcome depends on the virulence of the S. aureus strain, the type and concentration of toxins present in the PC, as well as the patience’s immune status (2,7). Despite implementation of multifaceted safety protocols, transfusion complications and sepsis are recurrent due to contamination with bacterial pathogens that can be missed during PC screening (4-6). Detection rates of PC testing with automated culture systems depend on the time between blood collection and PC sampling and the volume of PC sampled. Since the initial bacteria concentration in PCs may be very low (<1 CFU/mL), false-negative cultures at a rate of around 50% can be found even when both aerobic and anaerobic cultures are used (17). S. aureus is notable for producing toxins as one of its vital virulence determinants, and if present in PCs, these toxins can cause septic reactions, as previously reported (2,7). To our knowledge, this is the first report describing the presence of S. aureus superantigen toxin SEG in PCs involved in a septic transfusion reaction.
Superantigen exotoxins are important virulence factors produced by S. aureus; concentrations of less than 0.1 pg/mL are sufficient to cause fever, shock, and hypotension (14). Molecular analyses of superantigen production by S. aureus ATR-20003 and superantigen presence in the PC revealed that this isolate has the potential to produce five enterotoxins (SEs-G, M, N, O and SE-like U). These superantigens, which form the majority of the enterotoxin gene cluster (egc) gene repertoire, are prevalent in both community and clinical toxin-producing S. aureus isolates (18).
Although egc enterotoxins have not been associated with mortality in patients suffering from staphylococcal bacteremia, SEs-G, M, N and O are reported to elicit emetic responses (19). Earlier research suggests SEG involvement in toxic shock syndrome and in staphylococcal scarlet fever (20). Detection of the SEG toxin in both the supernatant from S. aureus ATR-20003 cultures, and the transfused PC, could explain the sharp rise in temperature and rigorous reaction experienced by the patient described in this case. A limitation of the investigation of this case was the lack of other antibodies that could be used to detect enterotoxins other than SEG. Multiple enterotoxin production by this organism is anticipated since its genome has genes that encode for five different enterotoxins.
Biofilm formation and slow growth dynamics displayed by S. aureus and other bacteria grown in PCs are associated with missed detection during screening with culture methods resulting in incidences of septic transfusion events (7,12). The false-negative BACT/ALERT result substantiates the increasing incidence of missed detection of S. aureus during PC screening with severe clinical consequences. As S. aureus can secrete enterotoxins into PCs, they could serve as biomarkers to anticipate clinical outcome of PC recipients, and therefore be part of essential testing during investigations of transfusion reactions involving S. aureus.
The transfusion physician communicated with the patient after the transfusion event and he did not disclose unconformities with the treatment received, and provided consent for the publication of this case report.
Acknowledgments
The authors are grateful to blood donors and netCAD Blood4Research Facility for blood collection and PC production for biofilm formation experiments. The authors thank Adriana Sferrazza, Akash Gupta and Marissa Laureano for their contributions to collecting information about the case.
Funding: The study was supported by Canadian Blood Services and Health Canada (grant # X000141). The views expressed herein do not necessarily represent those of the Canadian Federal government.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://aob.amegroups.com/article/view/10.21037/aob-21-38/rc
Peer Review File: Available at https://aob.amegroups.com/article/view/10.21037/aob-21-38/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-21-38/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ramirez-Arcos S, Goldman M. Bacterial Contamination. In: Kopko PM. AABB Transfusion Reactions. 5th ed. Bethesda, MD: AABB Press, 2021:115-64.
- Perpoint T, Lina G, Poyart C, et al. Two cases of fatal shock after transfusion of platelets contaminated by Staphylococcus aureus: role of superantigenic toxins. Clin Infect Dis 2004;39:e106-9. [Crossref] [PubMed]
- Kou Y, Pagotto F, Hannach B, et al. Fatal false-negative transfusion infection involving a buffy coat platelet pool contaminated with biofilm-positive Staphylococcus epidermidis: a case report. Transfusion 2015;55:2384-9. [Crossref] [PubMed]
- FDA. Fatalities Reported to FDA Following Blood Collection and Transfusion Annual Summary for FY2018 [monograph on the internet]. 2018. Available online: https://www.fda.gov/media/136907/download
- Levy JH, Neal MD, Herman JH. Bacterial contamination of platelets for transfusion: strategies for prevention. Crit Care 2018;22:271. [Crossref] [PubMed]
- Ramirez-Arcos S, Evans S, McIntyre T, et al. Extension of platelet shelf life with an improved bacterial testing algorithm. Transfusion 2020;60:2918-28. [Crossref] [PubMed]
- Loza-Correa M, Kou Y, Taha M, et al. Septic transfusion case caused by a platelet pool with visible clotting due to contamination with Staphylococcus aureus. Transfusion 2017;57:1299-303. [Crossref] [PubMed]
- Abela MA, Fenning S, Maguire KA, et al. Bacterial contamination of platelet components not detected by BacT/ALERT®. Transfus Med 2018;28:65-70. [Crossref] [PubMed]
- Haass KA, Sapiano MRP, Savinkina A, et al. Transfusion-Transmitted Infections Reported to the National Healthcare Safety Network Hemovigilance Module. Transfus Med Rev 2019;33:84-91. [Crossref] [PubMed]
- Brailsford SR, Tossell J, Morrison R, et al. Failure of bacterial screening to detect Staphylococcus aureus: the English experience of donor follow-up. Vox Sang 2018; [Crossref] [PubMed]
- Robillard P, Delage G, Itaj NK, et al. Use of hemovigilance data to evaluate the effectiveness of diversion and bacterial detection. Transfusion 2011;51:1405-11. [Crossref] [PubMed]
- Greco C, Mastronardi C, Pagotto F, et al. Assessment of biofilm-forming ability of coagulase-negative staphylococci isolated from contaminated platelet preparations in Canada. Transfusion 2008;48:969-77. [PubMed]
- Ono HK, Hirose S, Naito I, et al. The emetic activity of staphylococcal enterotoxins, SEK, SEL, SEM, SEN and SEO in a small emetic animal model, the house musk shrew. Microbiol Immunol 2017;61:12-6. [Crossref] [PubMed]
- Proft T, Fraser JD. Bacterial superantigens. Clin Exp Immunol 2003;133:299-306. [Crossref] [PubMed]
- Mastronardi C, Perkins H, Derksen P, et al. Evaluation of the BacT/ALERT 3D system for the implementation of in-house quality control sterility testing at Canadian Blood Services. Clin Chem Lab Med 2010;48:1179-87. [Crossref] [PubMed]
- Jacobs MR, Good CE, Lazarus HM, et al. Relationship between bacterial load, species virulence, and transfusion reaction with transfusion of bacterially contaminated platelets. Clin Infect Dis 2008;46:1214-20. [Crossref] [PubMed]
- Murphy WG, Foley M, Doherty C, et al. Screening platelet concentrates for bacterial contamination: low numbers of bacteria and slow growth in contaminated units mandate an alternative approach to product safety. Vox Sang 2008;95:13-9. [Crossref] [PubMed]
- Fischer AJ, Kilgore SH, Singh SB, et al. High Prevalence of Staphylococcus aureus Enterotoxin Gene Cluster Superantigens in Cystic Fibrosis Clinical Isolates. Genes (Basel) 2019;10:1036. [Crossref] [PubMed]
- Johler S, Giannini P, Jermini M, et al. Further evidence for staphylococcal food poisoning outbreaks caused by egc-encoded enterotoxins. Toxins (Basel) 2015;7:997-1004. [Crossref] [PubMed]
- Jarraud S, Cozon G, Vandenesch F, et al. Involvement of enterotoxins G and I in staphylococcal toxic shock syndrome and staphylococcal scarlet fever. J Clin Microbiol 1999;37:2446-9. [Crossref] [PubMed]
Cite this article as: Chi SI, Kumaran D, Zeller MP, Ramirez-Arcos S. Transfusion of a platelet pool contaminated with exotoxin-producing Staphylococcus aureus: a case report. Ann Blood 2022;7:43.


