
A narrative review of immune checkpoint mechanisms and current immune checkpoint therapy
Introduction
The immune system of an organism protects it against foreign invaders using three lines of defence. The first line of defence comprises the skin and mucous membrane, which act as a natural barrier; the second comprises macrophages and bactericidal substances, which act as the ‘vanguard’ of the immune response; and the third comprises the immune organs and cells, which generate specific immune responses. A series of immune checkpoints, with many signalling pathways, control the balance of effective immunity and self-tolerance. Stimulatory checkpoint pathways promote immune responses, while inhibitory checkpoint pathways inhibit immune responses (1).
However, some cancer cells and pathogens use various mechanisms to escape the immune system, such as by overactivating inhibitory immune checkpoints. Therefore, scientists have developed a range of immunotherapy treatments. Due to the demonstrated efficacy of several of these therapies, such as brake antibodies and genetically modified T cells, Science magazine deemed cancer immunotherapy the ‘Breakthrough of the Year’ in 2013 (2). In addition, the Nobel Prize in Physiology or Medicine was awarded to James P. Allison and Tasuku Honjo in 2018 for their development of cancer therapy using immune checkpoint inhibitors (ICIs) of cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) and programmed cell death 1 protein (PD-1) (https://www.nobelprize.org/prizes/medicine/2018/press-release/).
ICIs inhibit the overactivation of immune-checkpoint signalling pathways to a certain extent, thus promoting the immune response. ICIs have achieved prominent success in clinical trials, and the first ICI was approved in 2011 for the treatment of advanced melanoma. Currently, ICIs are also used to treat many other cancers, such as non-small cell lung cancer (NSCLC) and head and neck squamous cell carcinoma (3).
However, the monotherapeutic efficacy of ICIs is limited and resistance that develops after the initial clinical response is a major problem. However, new inhibitory and stimulatory pathways are good targets for immune checkpoint therapy (4,5) and combination therapy for cancer, thus providing patients with more treatment options.
In this review, we briefly summarise the mechanisms of the major immune checkpoint molecules in the immune system and the development of effective ICI drugs for clinical cancer therapy. Finally, we discuss the challenges and future directions of immune checkpoint cancer therapy, based on previous clinical studies. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aob.amegroups.com/article/view/10.21037/aob-21-3/rc).
Summary of immune modulatory mechanisms
The T-cell-mediated immune response involves multiple successive steps, including the positive and negative selection of T cells, which are then activated and proliferate in secondary lymphoid tissues. Subsequently, the T cells migrate to sites containing antigens and exhibiting inflammation, to execute direct effector functions (via cytokines, chemokines and ligands). A balance of the stimulatory and inhibitory signals of these steps is crucial for regulation of the immune response (6).
Co-stimulatory and inhibitory receptors and their ligands that regulate T-cell activation are generally not overexpressed in cancer tissues compared with normal tissues. However, inhibitory receptors and ligands that regulate T-cell effector functions are generally overexpressed in tumour cells or non-transformed cells in the tumour microenvironment. Soluble and membrane-bound receptor-ligand immune checkpoints are good targets for agonist antibodies (to synergistically stimulate pathways) or antagonist antibodies (to inhibit pathways). Therefore, unlike most antibodies that are approved for cancer therapy, these antibodies function by targeting lymphocyte receptors or their ligands to enhance their endogenous anti-tumour activity, rather than by directly targeting tumour cells (7).
Normally, immune checkpoints enable the immune system to respond to infections and malignancies, to protect normal tissues from damage. However, some of the immune checkpoint proteins are expressed in malignant cells, leading to immune dysregulation and the facilitation of tumour growth and expansion (8).
Here, we detail some of the most commonly studied immune checkpoint molecules including inhibitory and costimulatory molecules. We also discuss the role of immune checkpoint molecules that carry out opposite functions as membrane proteins or soluble proteins (such as Lymphocyte-activation gene 3, LAG3).
T cell-associated inhibitory molecules
CTLA-4
CTLA-4 is a type 1 transmembrane glycoprotein of the immunoglobulin superfamily and is similar to CD28. CTLA-4 consists of a signal peptide, an extracellular ligand-binding domain, a transmembrane domain and a cytoplasmic tail (9,10). CTLA-4 combines with oligomerised CD80 (B7-1) and CD86 (B7-2) ligands to deliver an inhibitory signal (11).
Mechanisms of CTLA-4
The interaction of CTLA-4 with CD80/86 inhibits T-cell activation via antagonism of CD28-mediated co-stimulation and suppresses interleukin-2 (IL-2) secretion and T-cell proliferation, but does not induce apoptosis (12-14). In addition, CTLA-4-expressing cells capture CD80/86, which induces the degradation of these ligands via trans-endocytosis (15).
One of the most significant biological features of CTLA-4 is its intracellular localisation and transport patterns. Most CTLA-4 resides in intracellular vesicles and endosomal compartments throughout the Golgi apparatus (16-18). In resting T cells, a small amount of CTLA-4 protein continues to circulate from the Golgi apparatus to the cell surface, after which it undergoes rapid endocytosis and lysosomal degradation (18). The cytoplasmic tail of CTLA-4 binds to the clathrin-associated adaptor proteins, activator protein-1 (AP-1) and AP-2/AP-50, to mediate its intracellular transport. In addition, the interaction between CTLA-4 and protein-T cell receptor-interacting molecule (TRIM) is important for the intracellular localisation and transport of CTLA-4 (12).
If T cell receptor (TCR) is engaged, CTLA-4 expression is induced, and intracellular vesicles containing CTLA-4 relocate to the immune synapse (16). The cytoplasmic tail of CTLA-4 is phosphorylated at Y165 by TCR-induced kinases; lymphocyte-specific protein tyrosine kinase (Lck) and f-chain-associated protein kinase 70 (ZAP-70), which disrupts the interaction of CTLA-4 and AP-2, and maintains cell-surface levels of CTLA-4 in the immune synapse (12). Notably, if the TCR signal is strong, more CTLA-4 accumulates in the immune synapse, which provides a dynamic and adjustable inhibitory signal (see Figure 1) (19).
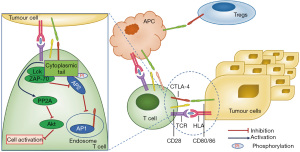
The ratio of B7 binding to CD28 or CTLA-4 determines whether T-cell activation is initiated or terminated (20). CTLA-4:B7 binding transmits the inhibitory signal in addition to blocking the stimulatory signal (21,22). Furthermore, there is evidence that CTLA-4 activates inhibitory signals through CD80/CD86 and induces the expression of indoleamine 2,3-dioxygenase (IDO) in antigen presenting cells (APCs), leading to localised tryptophan depletion and effector T cell inhibition, and the induction of regulatory T cells (Tregs) (23,24).
Researchers have demonstrated that CTLA-4 deletion in Tregs leads to spontaneous systemic lymphocyte proliferation, deadly autoimmune diseases and large amounts of immunoglobulin E production in mice with a resultant strong tumour immunity. CTLA-4 deletion in Tregs also impaired the ability of Tregs to inhibit the expression of CD80 and CD86 on dendritic cells (DCs), suggesting that Tregs may need CTLA-4 to inhibit the ability of APCs to recruit other T cells to suppress the immune response (25). In addition to the mechanisms described above, the depletion of Tregs is also considered to be a mechanism by which anti-CTLA-4 treatment functions in mouse tumour models (26).
CTLA-4 blockade appears to inhibit tumours via many different mechanisms. The primary mechanism is believed to involve direct competition of CTLA-4 with CD28 for binding to CD80/86 (27). This mainly occurs in tumour-draining lymph nodes, as tumour cells do not express B7 ligands, and APCs can cross-present tumour antigens to primary tumour-reactive T cells. In either case, tumour cell death requires the release of tumour cell antigens, such as neoantigens and tumour-associated antigens, which are then processed and presented by APCs. In the case of effective antigen presentation, CTLA-4 blockade enhances the co-stimulation of CD28 and thus activates T cell immune response (27).
The regulation of the TCR repertoire may also contribute to the therapeutic effects of CTLA-4 inhibition. Mechanistically, the absence of CTLA-4 may reduce the threshold of TCR ligation that is required to effectively activate T cells, because CTLA-4 usually weakens the intensity of the TCR signal (27). Thus, the blockade of CTLA-4 increases the mutual stimulation of T cells in a variety of ways, leading to more active tumour-reactive T cells.
Application
A seminal study showing that anti-CTLA-4 antibodies promoted anti-tumour immune responses in mouse tumour models (28) led to the clinical development of the anti-CTLA-4 antibody ipilimumab for cancer therapy, especially for melanoma treatment for Food and Drug Administration (FDA) approval. Significantly, ipilimumab was the first checkpoint inhibitor to gain regulatory approval for therapeutic use in the United States (29). Another antibody, tremelimumab, is a fully humanised IgG2 isotype monoclonal antibody (mAb) against CTLA-4. In a phase 1/2 clinical trial of melanoma patients, tremelimumab demonstrated an objective response rate (ORR) equivalent to standard chemotherapy (30).
Acute myeloid leukemia (AML) patients with the CTLA-4 CT60 AA genotype, which can generate more soluble form of CTLA-4 had increased of recurrence risk after conventional therapy and lower 3-year overall survival (31). 42% AML patients have effective immune responses with ipilimumab at 10 mg/kg dose, among them 3 responses were sustained for over 1 year (32). A phase I clinical study of the combination of ipilimumab and decitabine is in progress, which has achieved early clinical activity, particularly in relapsed/refractory (r/r) AML patients without transplanting (33).
New evidence suggests that anti-CTLA-4 treatment does not have a general effect on all T cells; rather, CTLA-4 blockade leads to the specific amplification of tumour neoantigen-specific CD8 T cells in the tumour microenvironment, rather than in secondary lymphoid organs (34). In fact, anti-CTLA-4 treatment can lead to the expansion of specific tumour-infiltrating T-cell populations, including the phenotypic depletion of CD8 T-cell subsets and PD-1+ICOS+TBET+ T helper type 1 (Th1)-like CD4 effector T-cell populations (where ICOS = inducible T-cell co-stimulator) (35). These populations appear to be different from typical Th1 cells, as the co-expression of ICOS and PD-1 are markers of T follicle-helper cells. These findings are supported by clinical observations of ICOS+ CD4 effector T-cells amplification after ipilimumab treatment of a variety of tumours (36-39), and after treatment with tremelimumab (40). Therefore, the amplification of ICOS+ CD4 effector T-cells may be used as a pharmacodynamic marker for anti-CTLA-4 therapy (41).
PD-1
PD-1, also called CD279, is an inhibitory receptor that belongs to the immunoglobulin superfamily. It is expressed on activated T cells, B cells, natural killer (NK) cells, DCs and activated monocytes (42).
PD-1 binds to two distinct ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC), which are members of the B7 protein family (42). In addition, PD-L1 has been reported to bind B7-1 (43). Experimental evidence suggests that the B7-1:PD-L1 interaction inhibits T cell function in a PD-1-independent manner (44). The specific functions of PD-L2 are less clear, as PD-L2-deficient mice have been reported to have increased (45) or decreased T-cell responses (46).
Mechanisms of PD-1
PD-1 are important in the maintenance of peripheral tolerance and the expected physiological response of T cells. Through interactions with PD-L1 and PD-L2, PD-1 regulates T-cell activation (47,48).
After stimulation of TCR, PD-1 is phosphorylated at tyrosine residues between the immunoreceptor tyrosine-based inhibition motif (ITIM) and immunoreceptor tyrosine-based switch motif (ITSM) in the cytoplasmic tail, resulting in the recruitment of phosphatases SHP-1 and SHP-2, which further dephosphorylate proximal signalling molecules downstream of TCR and CD28 (47-49). Point mutation studies indicate that the ITSM motif is necessary for inhibition by PD-1 (50,51). In addition, PD-1 ligation and recruitment to immune synapses appears necessary to mediate the inhibition of proximal TCR signals (51,52).
CTLA-4 and PD-1 inhibit Akt-induced T cell activation, thereby inhibiting the CD28-mediated induction of glucose uptake. However, their levels of inhibition are different (49). PD-1 inhibits Akt activation through the activation of proximal phosphoinositide 3-kinase (PI3K) by SHP-2, but CTLA-4 inhibits Akt activation through the activity of protein phosphatase 2A (PP2A) (see Figure 2) (49,53). Therefore, the combination of CTLA-4 and PD-1 results in the activation of partially overlapping yet distinct intracellular signalling pathways.
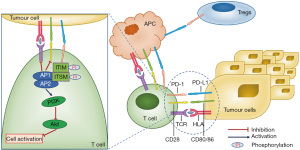
To maintain the immune homeostasis, Tregs induced by the PD-1 pathway maintains a high threshold for T cells activation to prevent autoimmunity. PD-L1 has the ability to promote the development and functions of Tregs in lymphoid system to avoid autoimmune responses, as it is expressed on both non-hematopoietic cells and hematopoietic cells. PD-L1 may also promote the de novo development of Tregs in tissues expressing transforming growth factor-β (54).
PD-1 blockade may induce tumour rejection by reactivating CD8 T cells, and increasing their functional activity and number. In addition, blocking the PD-1 signalling axis can prevent the attenuation of proximal TCR signalling, which is mediated by PD-1, to restore the activity of exhausted CD8 effectors. Therefore, although PD-L1 is continuously expressed in the tumour microenvironment, exhausted T cells can still be reactivated to produce an effective immune response (27).
In addition to restoring T-cell activity by regulating TCR signalling and gene expression, blocking PD-1 signalling can partially reverse relevant metabolic reprogramming, which mediates T-cell reactivation (55). In addition to directly blocking PD-1, immune-based tumour rejection can also be induced by antibodies targeting PD-L1. Due to the dominant expression of PD-L1, its blockade is considered to largely phenocopy the effect of PD-1 blockade. PD-L1 is induced by Th1 cytokines, such as interferon gamma (IFN-γ), while PD-L2 is induced by Th2 cytokines (56).
PD-1 signalling also plays a role in haematological neoplasia. AML blasts can down-regulate the expression of human leukocyte antigen, while promoting the overexpression of PD-L1 and other inhibitory T cell ligands. It can also promote the release of reactive oxygen species, IDO, arginase, and extracellular vesicles, which not only inhibit T and NK cells activities, but also mobilize Tregs and myeloid-derived suppressor cells (MDSCs), and promote macrophages transformation from M1 to M2 phenotype (57).
Application
In 2015, nivolumab and pembrolizumab gained regulatory approval for use as monotherapy for advanced recurrent NSCLC. Nivolumab, which was the first anti-PD-1 antibody to be approved, demonstrated promising therapeutic potential in phase I clinical trials, particularly for metastatic melanoma patients, NSCLC, and renal cell cancer (58). Pembrolizumab is a highly selective humanised IgG4 mAb against PD-1 (59). The success of pembrolizumab led to the rapid development of several other antibodies that block the PD-1/PD-L1 pathway for cancer therapy, such as pidilizumab (CureTech), MPDL3280A (Genentech), BMS-936559 (Bristol-Myers Squibb), and MEDI4736 (MedImmune/AstraZeneca).
Nivolumab and pembrolizumab have achieved an exciting overall response rate in r/r classical Hodgkin lymphoma (cHL) of 65–87% in phase I/II studies. Pembrolizumab has get shown an overall response rate in primary mediastinal large B-cell lymphoma (PMBL) of 48%. Nivolumab also acquired 36% and 40% ORR in diffused large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) patients, respectively. A phase II trial is ongoing with pembrolizumab in the treatment of DLBCL patients (60). For AML patients, nivolumab combined with azacitidine have achieved 33% ORR (57).
The combination of anti-CTLA-4 and anti-PD-1/PD-L1 mAbs in checkpoint-blockade cancer treatment can be both an opportunity and a challenge for immune therapy. However, progress has been made in combination therapy strategies in recent studies. Interim phase I results have been reported for nivolumab and ipilimumab as first-line therapy in patients with advanced NSCLC (NCT01454102), showing that this combination treatment had acceptable toxicity and activity in both PD-L1+ and PD-L1- patients (61). The combination of pembrolizumab and ipilimumab as second-line therapy in stage IIIB/IV NSCLC is currently being studied in the KEYNOTE-021 trial (NCT02039674), and has shown complete responses of 9% and partial responses of 45% for certain clinical aspects (61).
Lymphocyte activation gene-3 (LAG-3)
LAG-3 is the ligand of major histocompatibility complex (MHC) class II molecules and is thus a member of the immunoglobulin superfamily (62,63). It is expressed on activated NK cells and T cells, but not on resting T cells (62,64). MHC II, fibrinogen-like protein 1 (FGL1), galectin-3, LSECtin and α-synuclein are all ligands for LAG-3. FGL1 is an inhibitory ligand of LAG-3, independent of MHC II (65).
Mechanisms of LAG-3
LAG-3 is a negative regulator of T-cell activation. It is expressed as a co-receptor on T cells and modulates effector T-cell activity and Tregs suppressor activity (66).
The combination of LAG-3 and MHC class II, together with the CD40 and CD40L (the ligand of CD40) can affect the secretion of IL-12 and IFN-γ by APCs in vitro (67). Soluble LAG-3 can directly induce the production of Th1 cytokines or chemokines, such as macrophage-derived chemokine and thymus activation-regulated chemokine, by DCs. In response to these signals, mature DCs migrate to lymph nodes (68,69).
LAG-3 has also been found to be selectively upregulated on CD4+ Tregs (70). More recently, LAG-3 blockade (or genetic knockout) has been shown to affect the ability of conventional T cells to be suppressed by Tregs (71,72). Additionally, LAG-3 can maintain a tolerogenic state in CD8 cells, thus LAG-3 blocking antibodies augment CD8 T cell function in vivo, in the absence of CD4 T cells (73).
Application
The above preclinical studies have led to the development of two anti-LAG-3 molecules, BMS-986016 and LAG525, which are in clinical trials. BMS-986016 is an mAb antagonist of LAG-3 and is currently being assessed in five active clinical trials. These are phase I or II trials for the treatment of a variety of advanced solid tumours and haematological diseases. Most trials of BMS-986016 are being conducted in combination with a PD-1 inhibitor. LAG525 is being studied in a phase I/II clinical trial (NCT02460224), in combination with a PD-1 inhibitor, for the treatment of patients with advanced solid tumours (74).
IMP321 is a soluble form of LAG-3 that contains the first four extracellular domains of LAG-3, but lacks the transmembrane domain and intracellular region. It enhances tumour immune responses by upregulating co-stimulatory molecules and increasing IL-12 production. IMP321 has been assessed in two phase I clinical trials for the treatment of advanced renal cell carcinoma and pancreatic adenocarcinoma. It was shown to increase the number of tumour-reactive T cells, but no significant objective response (OR) was observed (75,76). IMP321 combined with paclitaxel for metastatic breast cancer treatment has also been assessed in a phase I clinical trial, in which an OR rate of 50% was observed (77). With this promising result, this study is currently recruiting metastatic breast cancer patients for a phase IIb clinical trial (NCT02614833) (78).
REGN3767, a fully human IgG4 antibody that in combination with a similar antibody, REGN2810, blocks the interaction of LAG3 and MHC II, has exhibited greater antitumour efficacy in preclinical tumour models than either antibody alone (79).
T cell immunoglobulin and mucin domain 3
T cell immunoglobulin and mucin domain 3 (TIM-3) is expressed on the surface of activated T cells, NK cells, and monocytes. Galectin-9 and carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) are the main ligands of TIM-3, among others (80,81).
Mechanisms of TIM-3
Upon binding to galectin-9 and several other ligands, TIM-3 facilitates peripheral immune tolerance by inducing Th1 cell death (82). TIM-3 is thought to be an important regulator of CD8+ T cell exhaustion in cancer (83). Recent studies have shown that TIM-3 is co-expressed with and interacts with CECAM1. This interaction is crucial to the regulatory function of TIM-3 (84).
Studies have shown that PD-1 is co-expressed with TIM-3 in tumour-infiltrating lymphocytes, suggesting a potential synergistic effect between these two checkpoint co-inhibitors. TIM-3+ PD-1+ tumour-infiltrating lymphocytes have an exhausted phenotype and secrete less IFN-γ, IL-2, and tumour necrosis factor alpha (TNF-α) (81,85).
Application
Preclinical studies indicate that TIM-3 inhibition enhances the function of effector T cells in the tumour microenvironment and increases their anti-tumour effect. This is especially true when TIM-3 blockage is combined with PD-1 inhibition. In mouse colorectal cancer models, the dual blockade of PD-1 and TIM-3 was more effective than monotherapies at inhibiting tumour growth (85). In addition, in a mouse model of head and neck squamous cell carcinoma, treatment with an anti-TIM-3 mAb resulted in decreased tumour growth, through the recovery of effector T-cell function (86).
A preclinical study of mice with lung adenocarcinoma found that TIM-3 expression increased in the tumours in the absence of PD-1 blockade. Subsequent TIM-3 blockade resulted in a significant survival advantage. The researchers then analysed two patients treated with PD-1 blockade and found that, with the increase in TIM-3 expression, a similar adaptive resistance pattern was observed (87). These promising data from preclinical studies have resulted in the development of two anti-TIM-3 mAbs, TSR-022 and MGB-453, which are currently being assessed in phase I clinical trials, in combination with PD-1 inhibitors, for treatment of patients with advanced solid tumours (NCT02817633, NCT02608268) (74).
T-cell immunoglobulin and ITIM domain
The T-cell immunoglobulin and ITIM domain (TIGIT) is part of the CD28-like family of receptors and is expressed in NK and T cells. TIGIT is composed of an extracellular IgV domain, a type 1 transmembrane region and a cytoplasmic tail. The cytoplasmic tail contains an ITIM domain and an immunoglobulin tail tyrosine (ITT)-like motif (88).
Mechanisms of TIGIT
TIGIT binds to CD155 to induce the phosphorylation of its cytoplasmic tail by Fyn and Lck. The phosphorylation of TIGIT causes its ITT-like motif to bind β-arrestin 2, which leads to the recruitment of SH2 domain containing inositol-5-phosphatase 1 (SHIP1) via the cytosolic adaptor growth-factor-receptor-bound protein 2 (Grb2). SHIP1 then blocks specific pathways, including the PI3K, mitogen-activated protein kinase (MAPK) and nuclear factor-κB signalling (NF-κB) pathways. The combined effects of TIGIT on these signalling pathways strongly inhibits the function of NK cells (89,90).
TIGIT has been shown to directly inhibit T cells in a cell-intrinsic manner, by targeting molecules in the TCR signalling pathway (91). As a result, TIGIT inhibits the activation, amplification and effector functions of T cells. In addition, TIGIT indirectly suppresses T-cell responses by interacting with CD155 on DCs (92).
TIGIT also contributes to immune suppression by promoting the function of Tregs. TIGIT is a direct target gene of forkhead box protein P3 (Foxp3), the master transcription factor in Tregs (93). Moreover, TIGIT expression levels correlate with markers of natural Tregs, rather than those of peripherally induced Tregs. TIGIT+ Tregs show enhanced demethylation in Treg-specific demethylation regions, compared to their TIGIT− counterparts, which leads to greater lineage stability. Further, TIGIT+ Tregs express higher levels of Treg markers, such as Foxp3, CD25 and CTLA-4, and the expression of TIGIT on Treg cells results in the upregulation of the inhibitory mediator, fibrinogen-like protein 2 (Fgl2), which enhances the suppressive function of TIGIT+ Tregs. Importantly, the TIGIT-dependent expression of Fgl2 results in the selective sparing of Th2 cell responses, while potently suppressing the responses of Th1 and Th17 cells (94).
CD226 (DNAM-1) and CD96 (Tactile) are known to bind TIGIT. CD226 transmits a positive co-stimulatory signal (95), whereas CD96 transmits an inhibitory signal (96). Similar to CTLA-4, TIGIT has a much higher affinity for ligands than CD226. Therefore, TIGIT blocks the interaction between CD226 and CD155, to inhibit co-stimulatory signals (92). However, TIGIT directly binds CD226 in cis, which disrupts the homodimerization of CD226 and inhibits its co-stimulatory function (97).
Application
In vitro studies and in vivo studies in mice have shown that dual blockade of TIGIT and PD-1 or TIM-3 has a synergistic effect on immune cell amplification, cytokine release, threshing, and the reversal of T cell exhaustion, resulting in tumour rejection and the induction of protective memory responses (98,99). Significantly, the expression levels of TIGIT in cells in the tumour microenvironment appear to be higher than the levels in peripheral cells, which should theoretically provide a more targeted, less toxic treatment method. In addition, TIGIT mainly appears to limit the competency of cytokines and the function of CD8 T cells, which accounts for its complementing the activity of other types of ICIs (82).
A phase I clinical trial (NCT03119428) is currently recruiting patients to assess the safety and potency of the anti-TIGIT mAb, OMP-31M32. However, the results of this trial are not yet available (8).
Tumour necrosis factor receptor 2 (TNFR2)
TNFR2 is a ligand of tumour necrosis factor alpha (TNFα), which has another ligand TNFR1. TNFα is an inflammatory cytokine with dual function. While it interacts with the widely expressed TNFR1 causing pro-inflammatory role and cell death, it also interacts with the limitedly expressed TNFR2 causing anti-inflammatory role and cell survival (100).
Mechanisms of TNFR2
The binding of TNF to TNFR2 induces the activation of NF-κB through NF-κB-inducible kinase (NIK), which further leads to phosphorylation of IKKα and p100 processing. TNFR2 also recruits the TRAF2-cIAP1-cIAP2 complex. cIAP has ubiquitin ligase activity, which can inhibit caspases and other apoptosis-inducing factors, thereby activating NF-κB/Rel and MAPK signalling pathways. TNFR2 signal transduction is mediated through RIPK1 and Etk respectively. RIPK1 triggers NF-κB through the IkB kinase (IKK) complex, which activates the expression of IL-2 and increases the transcription of several genes that are positively related to cell survival and proliferation. TNFR2-mediated phosphorylation of Etk can partially activate vascular endothelial growth factor receptor 2 (VEGFR2), then activates the PI3K/Akt signalling pathway to maintain cell survival and proliferation. It can also form a TNFR2-Etk-VEGFR2 complex by recruiting Etk to promote cell activation. In addition, it also enhances the phosphorylation of STAT5, which plays a key role in immunosuppression (101,102).
The stability, response to TCR stimulation, amplification and function of Tregs is enhanced when TNFR2 signal is activated. Researchers have demonstrated that blocking intrinsic membrane-bound TNF/TNFR2 signalling in CD4+ T-cells reduces IL-2 production and elevates Th17 differentiation which also correlated with enhanced STAT3 activity, increased ROR-γt level, and decreased STAT5 activity. The membrane-bound form mTNFR2 may change into the soluble form sTNFR2, which then binds TNF to inhibit the expression of IL-6 in inflammatory conditions (101).
Application
TNFR2 is expressed as an oncogene on many tumours. At present, the expression of TNFR2 has been determined in at least 25 tumours, such as ovarian cancer, colon cancer, multiple myeloma, cutaneous T-cell lymphoma (CTCL), et al. The tumour microenvironment cunningly recruits highly immunosuppressive TNFR2+ Tregs, thereby promoting tumour immune escape. The increased expression of TNFR2 gene in Tregs in patients with metastatic melanoma is related to the depletion of CD8+ T cells. TNFR2 knockout mice showed an enhanced immune response to tumours, and impaired tumour growth. This may be due to the lack of TNFR2+ Tregs, or the failure to develop systemic autoimmunity, or the decrease in the number of MDSCs and impaired function (103).
In vitro studies have shown that TNFR2 antagonist antibodies can inhibit the proliferation of ovarian cancer cells and tumour-related Tregs (104). Tumour cells expressing TNFR2 in advanced Sézary syndrome can be eliminated by TNFR2 antagonist antibodies, and TNFR2 antagonist antibodies can also kill TNFR2+ Tregs, adjust Treg/Teff ratio to normal level by inhibiting Tregs and Teff (103).
T cell-associated costimulatory molecules
4-1BB
4-1BB as a member of the TNFR superfamily 9, is a costimulatory molecule interaction with 4-1BBL, which is expressed in the surface of activated T cells and NK cells (105).
Mechanisms of 4-1BB
4-1BB recruits TNFR-associated factors (TRAF), TRAF1 and TRAF2 to form heterotrimers, through extracellular signal-regulated kinase (ERK) pathway, β-catenin and AKT pathways to enhance signal transduction. The main transcription factor NF-κB and MAPKs regulates 4-1BB signal to promote the production and secretion of IL-2 and IFN-γ, while promoting the survival and activation T cells by increasing the expression of antiapoptotic genes Bcl-xL and Bfl-1 (105,106).
However, the effect of 4-1BB on Tregs is still very controversial: 4-1BB agonist treatment can either inhibit the differentiation of conventional effector cells into Treg, while impacting the inhibitory effect of Tregs, or maintain Tregs amplification and inhibition ability (107,108).
4-1BB is also expressed in many non-T cells, such as DCs, monocytes, B cells, mast cells, NK cells and neutrophils. 4-1BB up-regulates B7-1 and B7-2, and increases the secretion of IL-6 and IL-12 in DC cells. The agonistic anti-4-1BB monoclonal antibody enhances the ability of DCs to stimulate T cell amplification and promotes the phosphorylation of STAT3 to enhance the CD8+ T cells response (109). After 4-1BB is triggered, NK cells upregulate 4-1BB and increase cytotoxic function, but 4-1BB activation on resting NK cells will reduce NK cells and impair the cytotoxic function of NK cells (105,110).
Application
CTL019 was the first CAR therapy to be approved by US Food and Drug Administration, which contains 4-1BB as an intracellular domain. Urelumab (BMS 663513) is a fully human IgG4 mAb, which is the first 4-1BB agonist antibody in clinical treatment. Utomilumab is a fully human IgG2 mAb, which has shown promising signs in patients with advanced solid tumours treated with monotherapy. The combination of utomilumab and pembrolizumab have shown 26% complete or partial responses (NCT02179918) (111).
CD27
CD27 is a member of TNFR superfamily, which is expressed in the early thymus of naïve CD4+ and CD8+ T cells (112).
Mechanisms of CD27
In the process of T cell activation, CD27 expression increased transiently, but after several rounds of T cells differentiation, the expression decreased (112). CD27 is also a marker of memory B cells, and expresses on NK cells (113,114). CD27 interacts with TRAF2 and TRAF5, while inducing the activation of NF-κB and MAPK8/JNK signalling pathway (115).
Application
Varlilumab is a CD27 agonist, which increases the production of chemokines, promotes T cells activation, and Tregs downregulation. It has shown 26% stable disease (SD) at 3 months in melanoma/renal cell carcinoma patients, and 1 partial response, 3 SD in 15 lymphoma patients. MDX-1203 is a CD70 mAb, which has shown 69% SD in patients (NCT00944905). SGN-75 is an antibody-drug conjugate of CD70 and monomethyl auristatin F, which also induces immune responses in renal cell carcinomas and lymphomas (NCT01015911) (116).
Inducible T-cell Co-stimulator (ICOS)
ICOS is a CD28 family protein, which is also called CD278 or AILIM, and mainly expressed in activated T cells. ICOS binding with a B7 family protein ICOSL plays the role of activating T cells (117,118).
Mechanisms of ICOS
ICOS binding with ICOSL, which is mainly expressed on APCs, through phosphoinositide 3-kinase (PI3K) signal pathway to enhance the function of Th1 and Th2 mainly by increasing the production of effector cytokines such as IL-4, IL-5, IL-10, IL-21, IFN γ and TNFα (119,120).
Application
T cells with high expression of ICOS had stronger immune response, upon using anti-CTLA-4 or anti-PD-1 mAbs to treat tumour of mice and patients, suggesting that ICOS maybe a useful target of tumour treatment (121-123). ICOS agonists are unlikely to be used for cancer monotherapy because they cannot directly induce a cytotoxic immune reaction independently. JTX-2011 is an ICOS agonist, which is used in combination with nivolumab in the phase 1 ICONIC clinical trial (NCT02904226). GSK3359609 is also a ICOS agonist, which is used in combination with pembrolizumab in phase I trials (NCT02723955) (116).
Methods
In this review, we mainly use PubMed (https://pubmed.ncbi.nlm.nih.gov/) to search literatures for two different parts: mechanisms and current therapy. Because the mechanisms are well-documented in early published articles, the literature year for this part is earlier, whereas the literature year for current therapy is chosen for recent 5 years mostly with the newer clinical outcome. Studies published in higher impact factor journals were prioritised.
The future of immune checkpoint therapy
Immune checkpoint therapy is an important component of immune therapy, and has enabled significant progress in cancer treatment. However, the use of immune checkpoint therapy alone may have limitations and induce side effects, such as autoimmune conditions. The combination of immune checkpoint therapy with other forms of immune therapy, such as chimeric antigen receptor-T cells (CAR-T), TCR-T cells or vaccines, or in combination therapy with multiple ICIs, are therefore promising approaches for cancer treatment, and several clinical studies are already underway to assess these strategies. Nevertheless, further research is needed to determine the anti-cancer mechanisms of ICIs, to enable the development of effective combination therapies. The similarities and differences in the mechanisms of different immune checkpoints must be established, as this will maximise the benefits of combination therapy, while minimising adverse effects.
In this review, we discuss the mechanisms and summarize current therapies of main immune checkpoint. Some mAbs of immune checkpoint are already applied in clinical trials or preclinical studies, which can provide some ideas to other scientists. A better understanding of the mechanisms of immune checkpoint will benefit the design of protein drugs, or combination therapy with immune checkpoint-blockade. Immune checkpoint therapy is considered as both an opportunity and a challenge for researchers. With the continued joint efforts of scientists and clinicians, further progress will be made in the future.
Acknowledgments
We would like to thank Armstrong-Hilton Ltd. for the help in polishing our manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aob.amegroups.com/article/view/10.21037/aob-21-3/rc
Peer Review File: Available at https://aob.amegroups.com/article/view/10.21037/aob-21-3/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-21-3/coif). YB reports employment relationship with Guangdong Xiangxue Life Sciences, Ltd. SP has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sharpe AH. Introduction to checkpoint inhibitors and cancer immunotherapy. Immunol Rev 2017;276:5-8. [Crossref] [PubMed]
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013;342:1432-3. [Crossref] [PubMed]
- Wang GX, Kurra V, Gainor JF, et al. Immune Checkpoint Inhibitor Cancer Therapy: Spectrum of Imaging Findings. Radiographics 2017;37:2132-44. [Crossref] [PubMed]
- Granier C, De Guillebon E, Blanc C, et al. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open 2017;2:e000213. [Crossref] [PubMed]
- Marin-Acevedo JA, Soyano AE, Dholaria B, et al. Cancer immunotherapy beyond immune checkpoint inhibitors. J Hematol Oncol 2018;11:8. [Crossref] [PubMed]
- Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol 2003;21:139-76. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Marin-Acevedo JA, Dholaria B, Soyano AE, et al. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol 2018;11:39. [Crossref] [PubMed]
- Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature 1987;328:267-70. [Crossref] [PubMed]
- Schwartz JC, Zhang X, Fedorov AA, et al. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature 2001;410:604-8. [Crossref] [PubMed]
- van der Merwe PA, Bodian DL, Daenke S, et al. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med 1997;185:393-403. [Crossref] [PubMed]
- Intlekofer AM, Thompson CB. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol 2013;94:25-39. [Crossref] [PubMed]
- Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med 1996;183:2533-40. [Crossref] [PubMed]
- Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med 1996;183:2541-50. [Crossref] [PubMed]
- Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 2011;332:600-3. [Crossref] [PubMed]
- Linsley PS, Bradshaw J, Greene J, et al. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity 1996;4:535-43. [Crossref] [PubMed]
- Leung HT, Bradshaw J, Cleaveland JS, et al. Cytotoxic T lymphocyte-associated molecule-4, a high-avidity receptor for CD80 and CD86, contains an intracellular localization motif in its cytoplasmic tail. J Biol Chem 1995;270:25107-14. [Crossref] [PubMed]
- Alegre ML, Noel PJ, Eisfelder BJ, et al. Regulation of surface and intracellular expression of CTLA4 on mouse T cells. J Immunol 1996;157:4762-70. [PubMed]
- Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity 2002;16:23-35. [Crossref] [PubMed]
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995;182:459-65. [Crossref] [PubMed]
- Fallarino F, Fields PE, Gajewski TF. B7-1 engagement of cytotoxic T lymphocyte antigen 4 inhibits T cell activation in the absence of CD28. J Exp Med 1998;188:205-10. [Crossref] [PubMed]
- Masteller EL, Chuang E, Mullen AC, et al. Structural analysis of CTLA-4 function in vivo. J Immunol 2000;164:5319-27. [Crossref] [PubMed]
- Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol 2004;172:4100-10. [Crossref] [PubMed]
- Park J, Kwon M, Shin EC. Immune checkpoint inhibitors for cancer treatment. Arch Pharm Res 2016;39:1577-87. [Crossref] [PubMed]
- Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008;322:271-5. [Crossref] [PubMed]
- Selby MJ, Engelhardt JJ, Quigley M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res 2013;1:32-42. [Crossref] [PubMed]
- Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov 2018;8:1069-86. [Crossref] [PubMed]
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271:1734-6. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Camacho LH, Antonia S, Sosman J, et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol 2009;27:1075-81. [Crossref] [PubMed]
- Huurman VA, Unger WW, Koeleman BP, et al. Differential inhibition of autoreactive memory- and alloreactive naive T cell responses by soluble cytotoxic T lymphocyte antigen 4 (sCTLA4), CTLA4Ig and LEA29Y. Clin Exp Immunol 2007;150:487-93. [Crossref] [PubMed]
- Davids MS, Kim HT, Bachireddy P, et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N Engl J Med 2016;375:143-53. [Crossref] [PubMed]
- Garcia JS, Werner L, Tomlinson BK, et al. Clinical and Immunologic Activity of Ipilimumab Following Decitabine Priming in Post-Allogeneic Transplant and Transplant-Naive Patients with Relapsed or Refractory Myelodysplastic Syndromes and Acute Myeloid Leukemia: A Multi-Center Phase 1, Two-Arm, Dose-Escalation Study. Blood 2019;134:2015. [Crossref]
- Fehlings M, Simoni Y, Penny HL, et al. Checkpoint blockade immunotherapy reshapes the high-dimensional phenotypic heterogeneity of murine intratumoural neoantigen-specific CD8+ T cells. Nat Commun 2017;8:562. [Crossref] [PubMed]
- Wei SC, Levine JH, Cogdill AP, et al. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell 2017;170:1120-33.e17. [Crossref] [PubMed]
- Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 2017;28:1368-79. [Crossref] [PubMed]
- Chen H, Liakou CI, Kamat A, et al. Anti-CTLA-4 therapy results in higher CD4+ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proc Natl Acad Sci U S A 2009;106:2729-34. [Crossref] [PubMed]
- Carthon BC, Wolchok JD, Yuan J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res 2010;16:2861-71. [Crossref] [PubMed]
- Liakou CI, Kamat A, Tang DN, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A 2008;105:14987-92. [Crossref] [PubMed]
- Vonderheide RH, LoRusso PM, Khalil M, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res 2010;16:3485-94. [Crossref] [PubMed]
- Ng Tang D, Shen Y, Sun J, et al. Increased frequency of ICOS+ CD4 T cells as a pharmacodynamic biomarker for anti-CTLA-4 therapy. Cancer Immunol Res 2013;1:229-34. [Crossref] [PubMed]
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [Crossref] [PubMed]
- Butte MJ, Keir ME, Phamduy TB, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007;27:111-22. [Crossref] [PubMed]
- Paterson AM, Brown KE, Keir ME, et al. The programmed death-1 ligand 1:B7-1 pathway restrains diabetogenic effector T cells in vivo. J Immunol 2011;187:1097-105. [Crossref] [PubMed]
- Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 2006;203:883-95. [Crossref] [PubMed]
- Shin T, Yoshimura K, Shin T, et al. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med 2005;201:1531-41. [Crossref] [PubMed]
- Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001;2:261-8. [Crossref] [PubMed]
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027-34. [Crossref] [PubMed]
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 2005;25:9543-53. [Crossref] [PubMed]
- Okazaki T, Maeda A, Nishimura H, et al. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A 2001;98:13866-71. [Crossref] [PubMed]
- Chemnitz JM, Parry RV, Nichols KE, et al. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 2004;173:945-54. [Crossref] [PubMed]
- Pentcheva-Hoang T, Chen L, Pardoll DM, et al. Programmed death-1 concentration at the immunological synapse is determined by ligand affinity and availability. Proc Natl Acad Sci U S A 2007;104:17765-70. [Crossref] [PubMed]
- Blair PJ, Riley JL, Levine BL, et al. CTLA-4 ligation delivers a unique signal to resting human CD4 T cells that inhibits interleukin-2 secretion but allows Bcl-X(L) induction. J Immunol 1998;160:12-5. [PubMed]
- Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 2010;236:219-42. [Crossref] [PubMed]
- Bengsch B, Johnson AL, Kurachi M, et al. Bioenergetic Insufficiencies Due to Metabolic Alterations Regulated by the Inhibitory Receptor PD-1 Are an Early Driver of CD8(+) T Cell Exhaustion. Immunity 2016;45:358-73. [Crossref] [PubMed]
- Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci U S A 2003;100:5336-41. [Crossref] [PubMed]
- Vago L, Gojo I. Immune escape and immunotherapy of acute myeloid leukemia. J Clin Invest 2020;130:1552-64. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Tanvetyanon T, Gray JE, Antonia SJ. PD-1 checkpoint blockade alone or combined PD-1 and CTLA-4 blockade as immunotherapy for lung cancer? Expert Opin Biol Ther 2017;17:305-12. [Crossref] [PubMed]
- Kline J, Godfrey J, Ansell SM. The immune landscape and response to immune checkpoint blockade therapy in lymphoma. Blood 2020;135:523-33. [Crossref] [PubMed]
- Xia B, Herbst RS. Immune checkpoint therapy for non-small-cell lung cancer: an update. Immunotherapy 2016;8:279-98. [Crossref] [PubMed]
- Triebel F, Jitsukawa S, Baixeras E, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med 1990;171:1393-405. [Crossref] [PubMed]
- Baixeras E, Huard B, Miossec C, et al. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med 1992;176:327-37. [Crossref] [PubMed]
- Huard B, Gaulard P, Faure F, et al. Cellular expression and tissue distribution of the human LAG-3-encoded protein, an MHC class II ligand. Immunogenetics 1994;39:213-7. [Crossref] [PubMed]
- Wang J, Sanmamed MF, Datar I, et al. Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell 2019;176:334-347.e12. [Crossref] [PubMed]
- Triebel F. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol 2003;24:619-22. [Crossref] [PubMed]
- Avice MN, Sarfati M, Triebel F, et al. Lymphocyte activation gene-3, a MHC class II ligand expressed on activated T cells, stimulates TNF-alpha and IL-12 production by monocytes and dendritic cells. J Immunol 1999;162:2748-53. [PubMed]
- Andreae S, Piras F, Burdin N, et al. Maturation and activation of dendritic cells induced by lymphocyte activation gene-3 (CD223). J Immunol 2002;168:3874-80. [Crossref] [PubMed]
- Buisson S, Triebel F. MHC class II engagement by its ligand LAG-3 (CD223) leads to a distinct pattern of chemokine and chemokine receptor expression by human dendritic cells. Vaccine 2003;21:862-8. [Crossref] [PubMed]
- Huang CT, Workman CJ, Flies D, et al. Role of LAG-3 in regulatory T cells. Immunity 2004;21:503-13. [Crossref] [PubMed]
- Sega EI, Leveson-Gower DB, Florek M, et al. Role of lymphocyte activation gene-3 (Lag-3) in conventional and regulatory T cell function in allogeneic transplantation. PLoS One 2014;9:e86551. [Crossref] [PubMed]
- Durham NM, Nirschl CJ, Jackson CM, et al. Lymphocyte Activation Gene 3 (LAG-3) modulates the ability of CD4 T-cells to be suppressed in vivo. PLoS One 2014;9:e109080. [Crossref] [PubMed]
- Grosso JF, Kelleher CC, Harris TJ, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest 2007;117:3383-92. [Crossref] [PubMed]
- Hahn AW, Gill DM, Pal SK, et al. The future of immune checkpoint cancer therapy after PD-1 and CTLA-4. Immunotherapy 2017;9:681-92. [Crossref] [PubMed]
- Wang-Gillam A, Plambeck-Suess S, Goedegebuure P, et al. A phase I study of IMP321 and gemcitabine as the front-line therapy in patients with advanced pancreatic adenocarcinoma. Invest New Drugs 2013;31:707-13. [Crossref] [PubMed]
- Brignone C, Escudier B, Grygar C, et al. A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma. Clin Cancer Res 2009;15:6225-31. [Crossref] [PubMed]
- Brignone C, Gutierrez M, Mefti F, et al. First-line chemoimmunotherapy in metastatic breast carcinoma: combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activity. J Transl Med 2010;8:71. [Crossref] [PubMed]
- Dirix L, Triebel F. AIPAC: a Phase IIb study of eftilagimod alpha (IMP321 or LAG-3Ig) added to weekly paclitaxel in patients with metastatic breast cancer. Future Oncol 2019;15:1963-73. [Crossref] [PubMed]
- Burova E, Hermann A, Dai J, et al. Preclinical Development of the Anti-LAG-3 Antibody REGN3767: Characterization and Activity in Combination with the Anti-PD-1 Antibody Cemiplimab in Human PD-1xLAG-3-Knockin Mice. Mol Cancer Ther 2019;18:2051-62. [Crossref] [PubMed]
- Sánchez-Fueyo A, Tian J, Picarella D, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol 2003;4:1093-101. [Crossref] [PubMed]
- Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015;27:450-61. [Crossref] [PubMed]
- Zhu C, Anderson AC, Schubart A, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 2005;6:1245-52. [Crossref] [PubMed]
- Fourcade J, Sun Z, Benallaoua M, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 2010;207:2175-86. [Crossref] [PubMed]
- Huang YH, Zhu C, Kondo Y, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 2015;517:386-90. [Crossref] [PubMed]
- Sakuishi K, Apetoh L, Sullivan JM, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 2010;207:2187-94. [Crossref] [PubMed]
- Liu JF, Ma SR, Mao L, et al. T-cell immunoglobulin mucin 3 blockade drives an antitumor immune response in head and neck cancer. Mol Oncol 2017;11:235-47. [Crossref] [PubMed]
- Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 2016;7:10501. [Crossref] [PubMed]
- Stengel KF, Harden-Bowles K, Yu X, et al. Structure of TIGIT immunoreceptor bound to poliovirus receptor reveals a cell-cell adhesion and signaling mechanism that requires cis-trans receptor clustering. Proc Natl Acad Sci U S A 2012;109:5399-404. [Crossref] [PubMed]
- Liu S, Zhang H, Li M, et al. Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell Death Differ 2013;20:456-64. [Crossref] [PubMed]
- Li M, Xia P, Du Y, et al. T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-γ production of natural killer cells via β-arrestin 2-mediated negative signaling. J Biol Chem 2014;289:17647-57. [Crossref] [PubMed]
- Joller N, Hafler JP, Brynedal B, et al. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol 2011;186:1338-42. [Crossref] [PubMed]
- Yu X, Harden K, Gonzalez LC, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol 2009;10:48-57. [Crossref] [PubMed]
- Zhang Y, Maksimovic J, Naselli G, et al. Genome-wide DNA methylation analysis identifies hypomethylated genes regulated by FOXP3 in human regulatory T cells. Blood 2013;122:2823-36. [Crossref] [PubMed]
- Joller N, Lozano E, Burkett PR, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 2014;40:569-81. [Crossref] [PubMed]
- Bottino C, Castriconi R, Pende D, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med 2003;198:557-67. [Crossref] [PubMed]
- Chan CJ, Martinet L, Gilfillan S, et al. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat Immunol 2014;15:431-8. [Crossref] [PubMed]
- Johnston RJ, Comps-Agrar L, Hackney J, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 2014;26:923-37. [Crossref] [PubMed]
- Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016;44:989-1004. [Crossref] [PubMed]
- Chauvin JM, Pagliano O, Fourcade J, et al. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J Clin Invest 2015;125:2046-58. [Crossref] [PubMed]
- Shamdani S, Uzan G, Naserian S. TNFα-TNFR2 signaling pathway in control of the neural stem/progenitor cell immunosuppressive effect: Different experimental approaches to assess this hypothetical mechanism behind their immunological function. Stem Cell Res Ther 2020;11:307. [Crossref] [PubMed]
- Yang S, Wang J, Brand DD, et al. Role of TNF-TNF Receptor 2 Signal in Regulatory T Cells and Its Therapeutic Implications. Front Immunol 2018;9:784. [Crossref] [PubMed]
- Leclerc M, Naserian S, Pilon C, et al. Control of GVHD by regulatory T cells depends on TNF produced by T cells and TNFR2 expressed by regulatory T cells. Blood 2016;128:1651-9. [Crossref] [PubMed]
- Torrey H, Khodadoust M, Tran L, et al. Targeted killing of TNFR2-expressing tumor cells and Tregs by TNFR2 antagonistic antibodies in advanced Sézary syndrome. Leukemia 2019;33:1206-18. [Crossref] [PubMed]
- Torrey H, Butterworth J, Mera T, et al. Targeting TNFR2 with antagonistic antibodies inhibits proliferation of ovarian cancer cells and tumor-associated Tregs. Sci Signal 2017;10:aaf8608. [Crossref] [PubMed]
- Chester C, Sanmamed MF, Wang J, et al. Immunotherapy targeting 4-1BB: mechanistic rationale, clinical results, and future strategies. Blood 2018;131:49-57. [Crossref] [PubMed]
- Lee DY, Choi BK, Lee DG, et al. 4-1BB signaling activates the t cell factor 1 effector/β-catenin pathway with delayed kinetics via ERK signaling and delayed PI3K/AKT activation to promote the proliferation of CD8+ T Cells. PLoS One 2013;8:e69677. [Crossref] [PubMed]
- Smith SE, Hoelzinger DB, Dominguez AL, et al. Signals through 4-1BB inhibit T regulatory cells by blocking IL-9 production enhancing antitumor responses. Cancer Immunol Immunother 2011;60:1775-87. [Crossref] [PubMed]
- Zhang P, Gao F, Wang Q, et al. Agonistic anti-4-1BB antibody promotes the expansion of natural regulatory T cells while maintaining Foxp3 expression. Scand J Immunol 2007;66:435-40. [Crossref] [PubMed]
- Wilcox RA, Chapoval AI, Gorski KS, et al. Cutting edge: Expression of functional CD137 receptor by dendritic cells. J Immunol 2002;168:4262-7. [Crossref] [PubMed]
- Choi BK, Kim YH, Kim CH, et al. Peripheral 4-1BB signaling negatively regulates NK cell development through IFN-gamma. J Immunol 2010;185:1404-11. [Crossref] [PubMed]
- Mayes PA, Hance KW, Hoos A. The promise and challenges of immune agonist antibody development in cancer. Nat Rev Drug Discov 2018;17:509-27. [Crossref] [PubMed]
- de Jong R, Loenen WA, Brouwer M, et al. Regulation of expression of CD27, a T cell-specific member of a novel family of membrane receptors. J Immunol 1991;146:2488-94. [PubMed]
- Klein U, Rajewsky K, Küppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med 1998;188:1679-89. [Crossref] [PubMed]
- Sugita K, Robertson MJ, Torimoto Y, et al. Participation of the CD27 antigen in the regulation of IL-2-activated human natural killer cells. J Immunol 1992;149:1199-203. [PubMed]
- Akiba H, Nakano H, Nishinaka S, et al. CD27, a member of the tumor necrosis factor receptor superfamily, activates NF-kappaB and stress-activated protein kinase/c-Jun N-terminal kinase via TRAF2, TRAF5, and NF-kappaB-inducing kinase. J Biol Chem 1998;273:13353-8. [Crossref] [PubMed]
- Burugu S, Dancsok AR, Nielsen TO. Emerging targets in cancer immunotherapy. Semin Cancer Biol 2018;52:39-52. [Crossref] [PubMed]
- Hutloff A, Dittrich AM, Beier KC, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 1999;397:263-6. [Crossref] [PubMed]
- Beier KC, Hutloff A, Dittrich AM, et al. Induction, binding specificity and function of human ICOS. Eur J Immunol 2000;30:3707-17. [Crossref] [PubMed]
- Gigoux M, Shang J, Pak Y, et al. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proc Natl Acad Sci U S A 2009;106:20371-6. [Crossref] [PubMed]
- Gigoux M, Lovato A, Leconte J, et al. Inducible costimulator facilitates T-dependent B cell activation by augmenting IL-4 translation. Mol Immunol 2014;59:46-54. [Crossref] [PubMed]
- Fan X, Quezada SA, Sepulveda MA, et al. Engagement of the ICOS pathway markedly enhances efficacy of CTLA-4 blockade in cancer immunotherapy. J Exp Med 2014;211:715-25. [Crossref] [PubMed]
- Kamphorst AO, Pillai RN, Yang S, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A 2017;114:4993-8. [Crossref] [PubMed]
- Tang C, Welsh JW, de Groot P, et al. Ipilimumab with Stereotactic Ablative Radiation Therapy: Phase I Results and Immunologic Correlates from Peripheral T Cells. Clin Cancer Res 2017;23:1388-96. [Crossref] [PubMed]
Cite this article as: Peng S, Bao Y. A narrative review of immune checkpoint mechanisms and current immune checkpoint therapy. Ann Blood 2022;7:33.


