
Novel insights in medical management of immune thrombocytopenia
Introduction
In this review we discuss the management of immune thrombocytopenia (ITP). More importantly, we provide new evidence on treatment modalities for cases failing first-line therapy. We appreciate that licensing and availability of the drugs will to an extent determine the selection of second-line therapies used. We are aware of the recently published guidance from the American Society of Hematology (ASH) and International Consensus Report (ICR) (1,2) and will therefore, in addition to the cornerstones of treatment, focus on additional developments following their recommendations; including new updates on rituximab, fostamatinib, decitabine, mycophenolate mofetil (MMF) and daratumumab. In this edition of Annals of Blood there are accompanying reviews covering the pathophysiology, pediatric ITP, pregnancy related ITP, intravenous immunoglobulin (IVIG), neonatal Fc receptor for IgG (FcRn) agonist and thrombopoietin (TPO)-agonist more in depth.
PubMed search
To build upon the literature incorporated in the ASH/ICR guidelines (1,2) on ITP, this review presents new publications from July 2018 to September 2020. Abstracts/titles from the electronic database PubMed were screened for the following search terms: immune thrombocytopenic purpura, idiopathic thrombocytopenic purpura, autoimmune thrombocytopenic purpura, autoimmune thrombocytopenia, immune thrombocytopenia, idiopathic thrombocytopenia, ITP. Corresponding MeSH terms were also searched. The following filters were applied: Clinical Study, Clinical Trial, Clinical Trial, Phase I, Clinical Trial, Phase II, Clinical Trial, Phase III, Clinical Trial, Phase IV, Comparative Study, Controlled Clinical Trial, Multicenter Study, Observational Study, Pragmatic Clinical Trial, Randomized Controlled Trial, Humans, English, from 2018/7/1 to 2020/11/12. Articles predominantly discussing therapies mentioned elsewhere in this journal issue have not been included (e.g., TPO-receptor agonists and pediatric ITP). Key papers in first-line management have been included, even if outside the above search.
First-line management ITP
The cornerstone of ITP management at presentation remains corticosteroids and IVIG
At presentation, the platelet count and the severity of the bleeding determine whether treatment will be either corticosteroids, IVIG, or a combination of the two (3). More on IVIG in ITP management can be found elsewhere in this issue. In severe life-threatening bleeding, platelets can be transfused, and even though the platelet count may not increment, they will aid in reducing the bleeding symptoms (4). They are not contraindicated as in thrombotic thrombocytopenic purpura (TTP) (5).
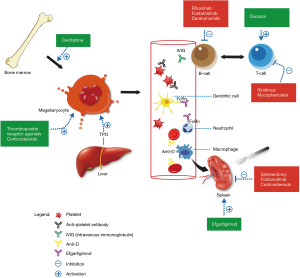
Corticosteroids work by decreasing platelet clearance and increasing platelet production (see Figure 1). Within this class of drugs, there is a choice between prednisolone and dexamethasone (6,7). There is no additional benefit of giving methylprednisolone over these two agents (8). Mithoowani et al. conducted a systematic review and meta-analysis of randomized trials between 1970 and 2016 that compared corticosteroids for long-term platelet count response (8). The primary endpoint was overall response (platelets >30×109/L) and complete response (platelets >100×109/L) at 6 months with high-dose dexamethasone compared with standard-dose prednisone. Nine trials comprising 1,138 participants were included. In adults, five trials compared 1–3 cycles of dexamethasone (40 mg per day for 4 days) with prednisolone (1 mg per kg) for 14–28 days followed by tapering. At 14 days the platelet count response was significantly higher in the dexamethasone group (79% vs. 59%) and fewer toxicities were reported. However, long-term response rates were similar.
Godeau et al. performed a randomised multicentre trial in which 122 adults with severe ITP were randomised to IVIG or methylprednisolone, and subsequently either oral prednisolone or placebo (9). In the IVIG group of 56 patients, the number of days in which the platelet count remained above 50 was 18 days, compared to 14 days in the 60 patients on methylprednisolone. Prednisolone was more effective compared to placebo for all short-term endpoints at day 21. From this it was concluded that IVIG and prednisolone were more effective than high dose methylprednisolone followed by oral prednisolone.
Similarly, Kim et al. retrospectively compared platelet responses and toxicities between IV methylprednisolone followed by oral prednisolone and IVIG with concomitant oral prednisone use (10). In this study 87 patients were enrolled, of which 77 were eligible for analysis. There was a statistically significant shorter time to complete response in the IVIG arm (6 days, range, 1–35 days) compared to methylprednisolone (13.5 days, range, 2–29 days). However, there was no significant difference in early response rate or long-term outcome between the two treatments (10). This variability in response rates is likely due to differences in assessment time following treatment initiation and variable response criteria, with Kim et al. following the response criteria set out by the International Working Group on ITP (9,10).
Pirunsarn et al. reported that maintenance therapy with prednisolone does not prolong relapse-free survival compared to observation alone (11). In their multicenter trial, 81 adult patients with either newly-diagnosed (53%) or relapsed ITP (57%) they randomized participants to either prednisolone 7.5 mg once daily or none. During the median follow-up of 42 weeks, the relapse rate was 22.7%, which on subgroup analysis, was best predicted by a history of relapsed ITP. There was no difference in relapse rate between the treatment vs. control cohorts at 20.5% vs. 25% respectively. Surprisingly, the mostly mild adverse events were similar in both groups.
Role of anti-D in the initial management of ITP
The mechanism of action of anti-D is unclear. It is believed that anti-D coats the red blood cells, and that these will saturate the Fcγ receptors on macrophages, rendering them unable to clear autoantibody-coated platelets (12). In a study comprising 20 adults with ITP, of which 9 were newly-diagnosed, 6 persistent and 5 chronic ITP, anti-D dosed at 50 µg/kg resulted in a median duration to response of 3 days (1–11 days). The overall response rate was 65%; in the newly-diagnosed 77%, persistent 50% and chronic 60% (13). The administration of anti-D can be cumbersome with a plethora of side effects including intravascular hemolysis leading to disseminated intravascular coagulation (DIC), renal failure, multiorgan failure and ultimately death (13). These adverse events are minimized by premedicating patients with corticosteroids (14). Given the mechanism of action of anti-D, splenectomized patients with ITP are unlikely to benefit from anti-D therapy (12). It should be noted that IV anti-D is not available in Europe (2). The withdrawal of licensing applications from the European Union by the manufacturers of WinRho® (15) came following risk benefit analysis with a particular focus on anti-D associated DIC (16).
Supportive treatment
Drugs affecting platelet function should be discontinued. In addition, anticoagulant therapy should be stopped in the majority of cases if the platelet count is below 50×109/L, however thrombotic risk and bleeding risk should be balanced and assessed.
With corticosteroids a proton pump inhibitor should be given, and close monitoring of the glucose levels is required. The ASH/ICR guidance (1,2) suggest giving acyclovir with high dose of dexamethasone.
Though the effect of antifibrinolytics has not been trialed in a randomized fashion in ITP, tranexamic acid (1 g every 6–8 hours orally) and epsilon-aminocaproic acid [1–5 g every 4–6 hours (maximum dose, 24 g/day)] are widely used in the acute setting where there are signs of bleeding (2). There is a theoretical risk of thrombosis in certain patient cohorts for example gastrointestinal (GI) bleeds secondary to liver disease, however in most other scenarios the benefits outweigh the potential risk.
Second-line treatments
If first-line therapy fails it is essential to ensure the diagnosis of ITP is correct. If not done already, investigations for other causes for thrombocytopenia should be revisited including a bone marrow aspirate. If the count remains in single figures, with or without clinical signs of bleeding, further therapies needs be initiated. The temporal sequencing of which will be dependent on the availability of the drug at the institute. The next group of drugs can be used in second-line treatment for ITP. To date only the TPOs and fostamatinib are licensed for chronic ITP defined as persistence for more than 12 months.
Rituximab and other anti-CD20s
B cells are the source of antibodies which are directed against platelet-surface glycoproteins in ITP (17). Rituximab is a monoclonal antibody directed against CD20 which is expressed only on mature B cell surface. Binding results in a rapid and deep, reversible B-cell depletion (18). There is a proportion of patients who do not respond to rituximab. This could be due to persisting long-lived plasma cells in the spleen, or bone marrow or abnormal activation of T-cells (19). In a systematic review including 313 adult patients with chronic ITP, an overall response rate of 62.5% and a complete response rate of 46.3% were found, with response duration between 2–48 months and a median duration of response of 10.5 months (20). The definition of complete response was a platelet count >150×109/L and overall response a platelet count >50×109/L).
Only two randomized controlled trials (RCTs) have been conducted for rituximab in ITP, which we will discuss next.
Investigating second-line management, the RITP trial (rituximab as second-line treatment for adult ITP) was a multicenter, randomized, double-blind, placebo-controlled trial in which corticosteroid-unresponsive adults were assigned to rituximab infusion 375 mg/m2 or placebo. A total of 112 patients were enrolled, of which 32 (58%) of the patients in the rituximab group and 37 (69%) in the placebo group had treatment failure at 78 weeks defined as the composite end point of splenectomy or meeting the criteria for splenectomy after 12 weeks if splenectomy was not done. However, rituximab did lengthen the median time to relapse from 7 to 36 week (21).
In a placebo-controlled trial comparing corticosteroids with or without Rituximab in the first-line setting the outcome did differ significantly but, not as much as had been expected. A total of 60 patients were recruited. After 6 months there was no difference between rituximab and placebo groups for the composite outcome of any platelet count below 50×109/L, significant bleeding or rescue therapy once standard treatment was stopped 66% vs. 81% (22).
The common dosing regimen is 375 mg/m2 weekly for four doses. However, there is evidence to suggest that a lower dose is equally effective (21-27). Alternative dosing regimens include: 1,000 mg on days 1 and 15 (27-29) and 100 mg weekly for 4 weeks (30,31). These showed responses lasting ≥1 year, with relapse rates in partial responders of 53% compared with 31% in complete responders (P<0.1) (32). The time to response is between 1–8 weeks (33). The predictors of fast responders to rituximab are females and younger patients <40 years (24). It is better when combined with corticosteroids (25). In most cases, in relapsed disease, re-treatment with rituximab is effective, especially in those who maintained remission preceding treatment for more than 12 months (34).
Rituximab is generally well tolerated and serious side effects are rare. The side effects causing the largest concern are progressive multifocal leukoencephalopathy (rare), reactivation of old infections, especially tuberculosis (TB) and hepatitis B, fatal infusion reactions and mucocutaneous reactions (35,36). Therefore, patients need to be screened for hepatitis B, and if present, treated concomitantly with the rituximab course. Severe side effects tend to occur in severely immunocompromised patients. In addition, repeated rituximab treatment, may result in hypogammaglobulinemia which needs to be monitored. Rituximab is effective in restoring the platelet count but does not result in reduction in bleeding episodes (37). There is no difference in the effect between rituximab or splenectomy as second-line therapy after IVIG and corticosteroids (38). Therefore, this is preferred over the irreversible splenectomy.
TPO receptor agonists
Although not licensed for newly-diagnosed ITP, they have been used in this setting. TPO receptor agonists are discussed in detail in other articles in this issue.
Fostamatinib
Fostamatinib is a spleen tyrosine kinase (Syk) tyrosine kinase inhibitor which has been recently approved by both the Food and Drug Administration (FDA) (39) and European Medicines Agency (EMA) (40) as a second-line treatment for chronic ITP and is currently under review by the National Institute for Health and Care Excellence (33,41). The Syk tyrosine kinase is expressed in hematopoietic cells, including macrophages, platelets and B-cells. In macrophages, activation of the tyrosine kinase leads via cytoskeletal re-arrangement to phagocytosis of antibody-coated platelets, but interestingly not reduce opsonization of bacteria (42,43). Syk also has a role in antibody generation, another angle through which it is an interesting target for ITP therapy (44,45). Syk has a role in platelet activation through the collagen receptor and integrin alfa-II-beta-3. Therefore, there were concerns about fostamatinib reducing platelet activation in patients already at risk of bleeding due to a reduced platelet count. However, in a single-dose study in healthy volunteers, it did not affect collagen or adenosine diphosphate-induced aggregation (43).
The drug was trialed simultaneously in two phase three randomized double-blind clinical trials. In total 150 patients were recruited. The median time from diagnosis was 8.5 years. Most participants had more than three therapies before entering the trial. Of note, most had received IVIG and corticosteroids; almost half had received a TPO-receptor agonist, one third had undergone splenectomy, treatment with rituximab or both (33). The median time to response was 15 days, which is comparable to the effect of TPO-receptor agonists but can take up to 2–8 weeks (33). The most common side effects were hypertension, nausea, diarrhea and neutropenia. This resulted in dose reductions in the study. The drug should be used at the starting dose for 1 month (100 mg BD), if the patient does not respond to this, then an increase is suggested to 150 mg BD. If the drug has not had any effect by 12 weeks, it should be discontinued (46). The benefit of fostamatinib over eltrombopag, which is also oral, is that there are no significant dietary restrictions, such as avoiding dairy products for 2 hours before and 4 hours after administration.
Decitabine
Decitabine is a hypomethylating agent which, at low dose, promotes cellular differentiation by increasing the number of mature polyploid megakaryocytes in vitro (47) and promoting platelet production and reducing bleeding risk in adults with ITP in vivo (48). It is licensed for the treatment of myelodysplastic syndrome and acute myeloid leukemia in the elderly, who are not suitable for conventional therapy. Zhou et al. conducted a recent multicenter study investigating the safety and efficacy of intravenous decitabine at low dose (3.5 mg/m2) for the treatment of refractory ITP in adults (48). Low dose decitabine was administered to 45 patients with refractory ITP as a 3-day course per cycle, for a total of 3 cycles with 4-week intervals between cycles. All patients previously received at least 3 previous therapies, with the majority receiving 4 or more. The median time to initial response was 28 days with a range of 2–10 weeks, which is longer than other treatment modalities for refractory ITP. Seventeen-point-seven-eight percent of patients achieved a complete response (platelet count at or over 100×109/L), and 33.33% achieved a partial response (no bleeding symptoms). A total of 86.96% of patients with an initial response achieved a sustained response (relapse-free without the need for additional therapy). Twenty-eight-point-eight-nine percent of patients experienced mild adverse effects including nausea, mild fever, diarrhea, constipation and mild increases in ALT/AST. Further RCTs with larger sample sizes are necessary to determine where in the treatment hierarchy decitabine would fit best as well as optimal dose and regime. However, Zhou et al. (48) describe the potential role for low-dose decitabine as an effective and safe therapy for ITP refractory to 3 or more therapies, regardless of splenectomy status.
Efgartigimod
Efgartigimod is a human IgG1 antibody Fc-fragment that binds to the FcRn with a higher affinity than the endogenous IgG autoantibodies implicated in the pathogenesis of ITP. As a result, these IgG auto-antibodies are degraded rapidly (49). The FcRn are expressed on antigen-presenting leukocytes such as neutrophils, dendritic cells and renal endothelial cells. Phase 2 studies of efgartigimod in the treatment of myasthenia gravis have shown the rapid reduction of pathogenic IgG (50). In a recent phase 2 study in 38 patients with predominantly refractory ITP, 4 weekly doses of efgartigimod intravenous infusion at either 5 or 10 mg/kg were compared to placebo (49). The rapid reduction of total IgG of up to 60.4% and 63.7% max mean change for low and high dose respectively, resulted in a subsequent response in platelet count as defined by the International Working Group for 38.5% of patients receiving efgartigimod at both low and high dose (49). Side effects included hematomas, petechiae, and purpura. It should be noted that this study was not adequately powered, and statistical significance was not tested as it was set up as exploratory study. One of the proposed mechanisms of action of IVIG is also through its effects on the FcRn (51), however given the limitations of IVIG, including issues surrounding supply, cost per treatment, and adverse effects (52), efgartigimod and other drugs targeting the FcRn (rozanolixizumab and nipocalimab) may offer a novel alternative (53). Further RCTs with larger sample sizes are required to compare the efficacy of efgartigimod compared to IVIG. An accompanying review in this journal will discuss efgartigimod.
MMF
MMF is a prodrug of mycophenolic acid (MFA) and acts as an immunosuppressive agent which directly inhibits T cell function. This occurs through its actions on the purine synthesis pathway and T cell activation steps (54). MMF is recommended as a second-line management option for ITP (1,3). MMF is generally well tolerated; mild and infrequent side effects include headache, backache, abdominal distension, anorexia, and nausea (3,54). Previous studies indicate dosing at 1 g/day (55).
The FLIGHT trial recently evaluated the efficacy of MMF in conjunction with corticosteroids compared to solely corticosteroids in the first-line management of ITP. In this multicenter, open label, randomized trial, 120 patients with ITP requiring first-line treatment were allocated either corticosteroids alone or in combination with MMF. The mean follow-up was 18 months. Patients receiving MMF with corticosteroids resulted in significantly less treatment failure 22% vs. 44% on solely corticosteroids (56). Though adverse effects were largely the same across both groups, certain aspects of the quality-of-life score were worse in the MMF group including physical function and fatigue. This study paves the way to consider it in first line, rather than second-line management.
Danazol
Danazol is an attenuated androgen, which may increase platelet production by antagonizing estrogen and through the immune modulation of T cells (57,58). Response rates vary between 10–70% with responses reached at 3–6 months across cohort studies (59). It appears that danazol, co-administered with corticosteroids, may achieve lower relapse rates compared to danazol alone (57). To date, no RCT has assessed the safety and efficacy of danazol vs. a control in the management of ITP, however one multicenter RCT has examined the safety and efficacy of recombinant human full length glycosylated TPO (rhTPO) with danazol compared to danazol alone in the treatment of 140 adults with refractory ITP. A combined approach with rhTPO and danazol was more effective in increasing mean maximal platelet count and achieved response in a shorter time compared to danazol (60). Both groups received danazol at 200 mg TDS; it should be noted that 26.2% of the danazol controls experienced adverse events (60). Danazol use should be used with caution in female patients due to virilizing side effects (59); patients should be made aware of side effects.
Other agents
For alemtuzumab, azathioprine, cyclosporin A, cyclophosphamide, hematopoietic stem cell transplantation (HSCT), dapsone, thalidomide and vinca-alkaloids there is no RCT data nor new evidence in adults since the updated ASH/ICR guidance (1,2), and therefore we will not further discuss them in this review. We will briefly discuss Daratumumab as there are new case reports.
Daratumumab
Daratumumab is a human immunoglobulin G1κ monoclonal antibody that targets CD38 (61). IV daratumumab is licensed for the treatment of refractory multiple myeloma, however recent case reports have highlighted potential utility in the management of refractory ITP (62). Two patients with myelodysplastic syndromes undergoing HSCT were treated with IV daratumumab at 16 mg/kg due to the development of prolonged severe thrombocytopenias following HSCT (62,63). Though a very small sample size, daratumumab has shown a sustained complete response at 16 months, without any bleeding symptoms clinically (62). A total of 7 post-transplant patients with refractory thrombocytopenias have shown successful responses to daratumumab (62). Further large-scale studies should be carried out, and even still it is not known whether these results would translate to primary ITP.
Splenectomy
The spleen and, to a lesser extent, the liver are the major organs where antibody-coated platelets are cleared by macrophages. Splenectomy is therefore an effective therapy in ITP. The effect of splenectomy can take 1–24 days (33). To be able to assess for the possibility of spontaneous remission or platelet count stabilization, splenectomy is only recommended after 12–24 months, unless it is a life-threatening emergency situation.
In 78% of the patients the response has been maintained for 10 years (64), and in 67% of the cases a relapse-free survival has been reported (65). It can be concluded from these numbers that up to 19% of patients have no response (65-67). The main factor associated with poor response is age ≥60 years, which has higher rates of relapse and postoperative complications (66). Given that a significant proportion of patients may observe non complete response and post-operative complications, pre-operative candidate selection is of great interest. In addition to age, splenic sequestration status may aid in the selection of patients for splenectomy. Using autologous 111 in-labelled platelet sequestration studies reveal adjusted odds ratios for complete response of 7.47 (at 1–3 months post splenectomy) and 4.85 (at 6–12 months post splenectomy) in patients with pure/predominantly splenic sequestration compared to mixed/hepatic sequestration (68).
Only two retrospective studies have compared splenectomy with rituximab (38,69). The first included 143 patients with ITP, of which 62 needed treatment and a further 30 required second-line therapy. Of these 19 received rituximab and 11 underwent a splenectomy. At baseline the platelet counts were similar. The splenectomy patients were younger. There was no difference in platelet count or relapse rates (38).
In a second, much larger collection of 105 patients; 43 were treated with rituximab and 62 were splenectomized (69). The mean follow-up was 3 and 8.4 years respectively. There was no difference between the two groups regarding the primary and clinical outcomes, defined as a composite: death from hemorrhage or from infection and hospitalization for bleeding or for infection. In the splenectomized group, the secondary outcomes were all better. These included overall mortality, hospitalization for bleeding, hospitalization for infection, as well as response and complete response (international definitions). There was a higher rate of response at 12 months in splenectomized patients (69).
Hammond et al. performed a comparative study in 218 patients with ITP, that relapsed after treatment with corticosteroids (70). As a second-line treatments, splenectomy provided a longer relapse free period than rituximab in second line (67.4% vs. 19.2%; P<0.001). Among patients who fail second-line treatment with splenectomy or rituximab, those who end up receiving sequential splenectomy-rituximab or rituximab-splenectomy therapy seem to derive similar benefit in the long term. Patients who receive rituximab after splenectomy have better results than those having rituximab before splenectomy.
Post-splenectomy there is a higher risk of thromboembolism and infections (e.g., pneumonia, meningitis, and septicemia) (71). These complications can occur at any time post procedure. An age ≥60 years is associated with higher complication rates. For example, in 73% of 39 patients, 3–7 days after splenectomy, CT scans portal vein or splenic vein clots were present, 80% of which had spontaneously disappeared at a repeat scan done around 42 days (72). Infection prevention consists of vaccinations ideally pre-splenectomy, prophylactic antibiotics and patient education about their increased risk for infections.
Conclusions
The management of ITP has evolved as greater therapeutic avenues have emerged including Syk inhibitors such as fostamatinib, FcRn agonists including efgartigimod, and TPO receptor agonists for example eltrombopag, romiplostim and avatrombopag. Though corticosteroids and IVIG remain the mainstay of first-line management, studies comparing efficacy of particular corticosteroids have had variable results, potentially due to the variability of how they quantified response. This highlights the importance of ITP studies following uniform criteria for measuring responses, such as those suggested by the International Working Group. Studies such as the FLIGHT trial into the role of previously second-line therapies for chronic ITP, may indeed play a role in the management of primary ITP as a first-line approach in conjunction with corticosteroids or IVIG. As highlighted in this review, there is a need for large scale RCTs, particularly for cheaper and more widely available management options.
Acknowledgments
The authors would like to acknowledge John W. Semple and Rick Kaur for inviting this article to this series on ITP.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (John W. Semple and Rick Kapur) for the series “Treatment of Immune Thrombocytopenia (ITP)” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob-21-7). The series “Treatment of Immune Thrombocytopenia (ITP)” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv 2019;3:3829-66. [Crossref] [PubMed]
- Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv 2019;3:3780-817. [Crossref] [PubMed]
- Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010;115:168-86. [Crossref] [PubMed]
- Piel-Julian ML, Mahévas M, Germain J, et al. Risk factors for bleeding, including platelet count threshold, in newly diagnosed immune thrombocytopenia adults. J Thromb Haemost 2018;16:1830-42. [Crossref] [PubMed]
- Zheng XL, Vesely SK, Cataland SR, et al. ISTH guidelines for treatment of thrombotic thrombocytopenic purpura. J Thromb Haemost 2020;18:2496-502. [Crossref] [PubMed]
- Shulman NR, Weinrach RS, Libre EP, et al. The role of the reticuloendothelial system in the pathogenesis of idiopathic thrombocytopenic purpura. Trans Assoc Am Physicians 1965;78:374-90. [PubMed]
- Gernsheimer T, Stratton J, Ballem PJ, et al. Mechanisms of Response to Treatment in Autoimmune Thrombocytopenic Purpura. N Engl J Med 1989;320:974-80. [Crossref] [PubMed]
- Mithoowani S, Gregory-Miller K, Goy J, et al. High-dose dexamethasone compared with prednisone for previously untreated primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol 2016;3:e489-96. [Crossref] [PubMed]
- Godeau B, Chevret S, Varet B, et al. Intravenous immunoglobulin or high-dose methylprednisolone, with or without oral prednisone, for adults with untreated severe autoimmune thrombocytopenic purpura: A randomised, multicentre trial. Lancet 2002;359:23-9. [Crossref] [PubMed]
- Kim CH, Choi YS, Moon JY, et al. Methylprednisolone versus intravenous immune globulin as an initial therapy in adult primary immune thrombocytopenia. Korean J Intern Med 2019;34:383-9. [Crossref] [PubMed]
- Pirunsarn A, Kijrattanakul P, Chamnanchanunt S, et al. A Randomized Multicenter Trial Comparing Low-Dose Prednisolone Versus Observation for Prevention of Recurrences in Adult Immune Thrombocytopenia. Clin Appl Thromb Hemost 2018;24:867-73. [Crossref] [PubMed]
- Lazarus AH, Crow AR. Mechanism of action of IVIG and anti-D in ITP. Transfus Apher Sci 2003;28:249-55. [Crossref] [PubMed]
- Naithani R, Kumar R, Mahapatra M, et al. Efficacy and safety of anti-D for treatment of adults with immune thrombocytopenia. Platelets 2009;20:525-7. [Crossref] [PubMed]
- Despotovic JM, Lambert MP, Herman JH, et al. RhIG for the treatment of immune thrombocytopenia: consensus and controversy (CME). Transfusion 2012;52:1126-36; quiz 1125. [Crossref] [PubMed]
- Velthove KJ, Strengers PFW. Blood, blood components, plasma, and plasma products. In: Aronson JK. editor. Side Effects of Drugs Annual. Elsevier B.V., 2012:509-29.
- Gaines AR. Disseminated intravascular coagulation associated with acute hemoglobinemia or hemoglobinuria following Rh(0)(D) immune globulin intravenous administration for immune thrombocytopenic purpura. Blood 2005;106:1532-7. [Crossref] [PubMed]
- Arnold DM, Vrbensky JR, Karim N, et al. The effect of rituximab on anti-platelet autoantibody levels in patients with immune thrombocytopenia. Br J Haematol 2017;178:302-7. [Crossref] [PubMed]
- Roll P, Palanichamy A, Kneitz C, et al. Regeneration of B cell subsets after transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Arthritis Rheum 2006;54:2377-86. [Crossref] [PubMed]
- Cooper N, Stasi R, Cunningham-Rundles S, et al. Platelet-associated antibodies, cellular immunity and FCGR3a genotype influence the response to rituximab in immune thrombocytopenia. Br J Haematol 2012;158:539-47. [Crossref] [PubMed]
- Arnold DM, Dentali F, Crowther MA, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med 2007;146:25-33. [Crossref] [PubMed]
- Ghanima W, Khelif A, Waage A, et al. Rituximab as second-line treatment for adult immune thrombocytopenia (the RITP trial): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015;385:1653-61. [Crossref] [PubMed]
- Arnold DM, Heddle NM, Carruthers J, et al. A pilot randomized trial of adjuvant rituximab or placebo for nonsplenectomized patients with immune thrombocytopenia. Blood 2012;119:1356-62. [Crossref] [PubMed]
- Miyakawa Y, Katsutani S, Yano T, et al. Efficacy and safety of rituximab in Japanese patients with relapsed chronic immune thrombocytopenia refractory to conventional therapy. Int J Hematol 2015;102:654-61. [Crossref] [PubMed]
- Marangon M, Vianelli N, Palandri F, et al. Rituximab in immune thrombocytopenia: gender, age, and response as predictors of long-term response. Eur J Haematol 2017;98:371-7. [Crossref] [PubMed]
- Červinek L, Černá O, Čaniga M, et al. Efficacy of rituximab in primary immune thrombocytopenia: An analysis of adult pretreated patients from everyday hematological practice. Int J Hematol 2012;96:594-9. [Crossref] [PubMed]
- Pasa S, Altintas A, Cil T, et al. The efficacy of rituximab in patients with splenectomized refractory chronic idiopathic thrombocythopenic purpura. J Thromb Thrombolysis 2009;27:329-33. [Crossref] [PubMed]
- Khellaf M, Charles-Nelson A, Fain O, et al. Safety and efficacy of rituximab in adult immune thrombocytopenia: Results from a prospective registry including 248 patients. Blood 2014;124:3228-36. [Crossref] [PubMed]
- Mahévas M, Ebbo M, Audia S, et al. Efficacy and safety of rituximab given at 1,000 mg on days 1 and 15 compared to the standard regimen to treat adult immune thrombocytopenia. Am J Hematol 2013;88:858-61. [Crossref] [PubMed]
- Tran H, Brighton T, Grigg A, et al. A multi-centre, single-arm, open-label study evaluating the safety and efficacy of fixed dose rituximab in patients with refractory, relapsed or chronic idiopathic thrombocytopenic purpura (R-ITP1000 study). Br J Haematol 2014;167:243-51. [Crossref] [PubMed]
- Zaja F, Vianelli N, Volpetti S, et al. Low-dose rituximab in adult patients with primary immune thrombocytopenia. Eur J Haematol 2010;85:329-34. [Crossref] [PubMed]
- Zaja F, Volpetti S, Chiozzotto M, et al. Long-term follow-up analysis after rituximab salvage therapy in adult patients with immune thrombocytopenia. Am J Hematol 2012;87:886-9. [Crossref] [PubMed]
- Patel VL, Mahévas M, Lee SY, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood 2012;119:5989-95. [Crossref] [PubMed]
- Connell NT, Berliner N. Fostamatinib for the treatment of chronic immune thrombocytopenia. Blood 2019;133:2027-30. [Crossref] [PubMed]
- Lucchini E, Zaja F, Bussel J. Rituximab in the treatment of immune thrombocytopenia: What is the role of this agent in 2019? Haematologica 2019;104:1124-35. [Crossref] [PubMed]
- Genentech. Rituxan highlights of prescribing information 2018 Oct [cited 2020 Dec 21]. Available online: www.fda.gov/medwatch
- Roche Registration GmbH. MabThera summary of product characteristics. 2018 Sep. Available online: https://www.ema.europa.eu/en/documents/product-information/mabthera-epar-product-information_en.pdf
- Chugh S, Darvish-Kazem S, Lim W, et al. Rituximab plus standard of care for treatment of primary immune thrombocytopenia: A systematic review and meta-analysis. Lancet Haematol 2015;2:e75-81. [Crossref] [PubMed]
- Al Askar AS, Shaheen NA, Al Zahrani M, et al. Splenectomy vs. rituximab as a second-line therapy in immune thrombocytopenic purpura: a single center experience. Int J Hematol 2018;107:69-74. [Crossref] [PubMed]
- FDA approves fostamatinib tablets for ITP | FDA [cited 2020 Dec 21]. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fostamatinib-tablets-itp
- Tavlesse | European Medicines Agency [cited 2020 Dec 21]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tavlesse
- Project information | Fostamatinib for treating persistent or chronic immune thrombocytopenia [ID1087] | Guidance | NICE [cited 2020 Dec 21]. Available online: https://www.nice.org.uk/guidance/indevelopment/gid-ta10387
- Newland A, Lee EJ, McDonald V, et al. Fostamatinib for persistent/chronic adult immune thrombocytopenia. Immunotherapy 2018;10:9-25. [Crossref] [PubMed]
- Braselmann S, Taylor V, Zhao H, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther 2006;319:998-1008. Erratum in: J Pharmacol Exp Ther 2013 May;345(2):326. [Crossref] [PubMed]
- Geahlen RL. Getting Syk: spleen tyrosine kinase as a therapeutic target. Trends Pharmacol Sci 2014;35:414-22. [Crossref] [PubMed]
- Ozaki N, Suzuki S, Ishida M, et al. Syk-dependent signaling pathways in neutrophils and macrophages are indispensable in the pathogenesis of anti-collagen antibody-induced arthritis. Int Immunol 2012;24:539-50. [Crossref] [PubMed]
- FDA, Rigel Pharmaceuticals. TAVALISSE HIGHLIGHTS OF PRESCRIBING INFORMATION 2018 Apr [cited 2020 Dec 21]. Available online: www.fda.gov/medwatch
- Zhou H, Hou Y, Liu X, et al. Low-dose decitabine promotes megakaryocyte maturation and platelet production in healthy controls and immune thrombocytopenia. Thromb Haemost 2015;113:1021-34. [Crossref] [PubMed]
- Zhou H, Qin P, Liu Q, et al. A prospective, multicenter study of low dose decitabine in adult patients with refractory immune thrombocytopenia. Am J Hematol 2019;94:1374-81. [Crossref] [PubMed]
- Newland AC, Sánchez-González B, Rejtő L, et al. Phase 2 study of efgartigimod, a novel FcRn antagonist, in adult patients with primary immune thrombocytopenia. Am J Hematol 2020;95:178-87. [Crossref] [PubMed]
- Howard JF, Bril V, Burns TM, et al. Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology 2019;92:e2661-73. [Crossref] [PubMed]
- Jin F, Balthasar JP. Mechanisms of intravenous immunoglobulin action in immune thrombocytopenic purpura. Hum Immunol 2005;66:403-10. [Crossref] [PubMed]
- Hsia CC, Liu Y, Eckert K, et al. Intravenous Immunoglobulin (IVIg) Utilization in Immune Thrombocytopenia (ITP): A Multi-Center, Retrospective Review. Drugs Real World Outcomes 2015;2:35-42. [Crossref] [PubMed]
- Gable KL, Guptill JT. Antagonism of the Neonatal Fc Receptor as an Emerging Treatment for Myasthenia Gravis. Front Immunol 2020;10:3052. [Crossref] [PubMed]
- Provan D, Moss AJ, Newland AC, et al. Efficacy of mycophenolate mofetil as single-agent therapy for refractory immune thrombocytopenic purpura. Am J Hematol 2006;81:19-25. [Crossref] [PubMed]
- Taylor A, Neave L, Solanki S, et al. Mycophenolate mofetil therapy for severe immune thrombocytopenia. Br J Haematol 2015;171:625-30. [Crossref] [PubMed]
- Bradbury C, Greenwood R, Pell J, et al. A Multicentre Randomised Trial of First Line Treatment Pathways for Newly Diagnosed Immune Thrombocytopenia: Standard Steroid Treatment Versus Combined Steroid and Mycophenolate. the Flight Trial. In: 62nd ASH Annual Meeting and Exposition. ASH, 2020.
- Liu W, Gu X, Fu R, et al. The Effect of Danazol in Primary Immune Thrombocytopenia: An Analysis of a Large Cohort from a Single Center in China. Clin Appl Thromb Hemost 2016;22:727-33. [Crossref] [PubMed]
- Mylvaganam R, Ahn YS, Harrington WJ, et al. Immune modulation by danazol in autoimmune thrombocytopenia. Clin Immunol Immunopathol 1987;42:281-7. [Crossref] [PubMed]
- Cuker A, Neunert CE. How I treat refractory immune thrombocytopenia. Blood 2016;128:1547-54. [Crossref] [PubMed]
- Wang S, Yang R, Zou P, et al. A multicenter randomized controlled trial of recombinant human thrombopoietin treatment in patients with primary immune thrombocytopenia. Int J Hematol 2012;96:222-8. [Crossref] [PubMed]
- McKeage K. Daratumumab: First Global Approval. Drugs 2016;76:275-81. [Crossref] [PubMed]
- Migdady Y, Ediriwickrema A, Jackson RP, et al. Successful treatment of thrombocytopenia with daratumumab after allogeneic transplant: a case report and literature review. Blood Adv 2020;4:815-8. [Crossref] [PubMed]
- Neylon AJ, Saunders PWG, Howard MR, et al. Clinically significant newly presenting autoimmune thrombocytopenic purpura in adults: A prospective study of a population-based cohort of 245 patients. Br J Haematol 2003;122:966-74. [Crossref] [PubMed]
- Chater C, Terriou L, Duhamel A, et al. Reemergence of splenectomy for ITP second-line treatment? Ann Surg 2016;264:772-7. [Crossref] [PubMed]
- Vianelli N, Palandri F, Polverelli N, et al. Splenectomy as a curative treatment for immune thrombocytopenia: A retrospective analysis of 233 patients with a minimum follow up of 10 years. Haematologica 2013;98:875-80. [Crossref] [PubMed]
- Park YH, Yi HG, Kim CS, et al. Clinical outcome and predictive factors in the response to splenectomy in elderly patients with primary immune thrombocytopenia: A multicenter retrospective study. Acta Haematologica 2016;135:162-71. [Crossref] [PubMed]
- Tada K, Ohta M, Saga K, et al. Long-term outcomes of laparoscopic versus open splenectomy for immune thrombocytopenia. Surg Today 2018;48:180-5. [Crossref] [PubMed]
- Sarpatwari A, Provan D, Erqou S, et al. Autologous 111In-labelled platelet sequestration studies in patients with primary immune thrombocytopenia (ITP) prior to splenectomy: A report from the United Kingdom ITP Registry. Br J Haematol 2010;151:477-87. [Crossref] [PubMed]
- Moulis G, Sailler L, Sommet A, et al. Rituximab versus splenectomy in persistent or chronic adult primary immune thrombocytopenia: An adjusted comparison of mortality and morbidity. Am J Hematol 2014;89:41-6. [Crossref] [PubMed]
- Hammond WA, Vishnu P, Rodriguez EM, et al. Sequence of Splenectomy and Rituximab for the Treatment of Steroid-Refractory Immune Thrombocytopenia: Does It Matter? Mayo Clin Proc 2019;94:2199-208. [Crossref] [PubMed]
- Kristinsson SY, Gridley G, Hoover RN, et al. Long-term risks after splenectomy among 8,149 cancer-free American veterans: A cohort study with up to 27 years follow-up. Haematologica 2014;99:392-8. [Crossref] [PubMed]
- Morbieu C, Brunetti F, Baranès L, et al. Systematic detection of portal or splenic vein thrombosis after splenectomy for immune cytopenia. Am J Hematol 2018;93:E170-2. [Crossref] [PubMed]
Cite this article as: Hanif M, Sivapalaratnam S, Provan D. Novel insights in medical management of immune thrombocytopenia. Ann Blood 2021;6:8.


