
Cancer immunotherapy using T-cell receptor engineered T cell
Introduction
Cancer has become a main public health issue and one of the major causes of death in the world. In 2018, available data indicated that there were estimated 18.1 million new cancer cases and 9.6 million cancer deaths. Lung cancer was the leading cause of cancer death (18.4%), which was followed by colorectum cancer (9.2%), stomach cancer (8.2%), liver cancer (8.2%), breast cancer (6.6%) and esophagus cancer (5.3%) (1). Both in developing and developed countries, malignancies, especially the late stage diseases, consume a lot of human and financial resources, but unfortunately, are still very difficult to be cured.
Traditionally, surgery, chemotherapy, and radiotherapy (RT) have developed as three pillars of cancer treatments. But big wounds, drug resistance and tumor relapse demonstrate the drawback of traditional cancer treatments. On the other hand, immunotherapy, which prevents tumor formation or suppresses its growth by reassembling the immune system intrinsic functions, is becoming a more and more reliable cancer treatment and forming a new pillar over the past two decades. But the biggest challenge we are facing in the immunotherapy is that the impact of central and peripheral immune tolerance can be utilized by being targeted tumors to hamper immune effector cells. There are numerous strategies for carrying out immunotherapies to overcome the immune tolerance, such as therapeutic vaccines, immune checkpoint blockades, tumor microenvironment (TM) regulation strategies or adoptive cellular therapy (ACT) (2).
ACTs have shown promising results in clinical investigations. To date, various ACTs, such as lymphokine-activated killer (LAK) cells (3), tumor-infiltrating lymphocytes (TILs) (4), cytokine-induced killer (CIK) cells (5)/cytokine activated T-cells (CATs) (6), γδ T cells, natural killer (NK) cells, chimeric antigen receptor-modified T cell therapy (CAR-T) (7) and T-cell receptor engineered T cell therapy (TCR-T) (8) have been developed and tested in clinics. As a passive immunotherapy, ACT processes may involve sorting immune cells from patients or healthy people, expanding the cells ex vivo, then infusing the bulk immune-cell populations into patients to treat the cancers. Relying on tumor-specific T cell, some of the ACT approaches are becoming potentially powerful treatments for cancer, and the direct examples are two US FDA recently granted CAR-T products, Kymriah for treating acute lymphoblastic leukemia (ALL) (9,10), and Yescarta for treating B-cell lymphoma (11,12).
The development of ACT is deeply related to clinic needs, and its evolution relies on tremendous discoveries in immunology in the past decades. Since ACT is such a complex therapy, and has demonstrated enormous potential for treating cancers in clinic practice, we believe that a collective summary of the development, key questions, critical modifications, should be helpful for improving the technology. In this review, we will summarize the history of ACT, and focus on TCR-T therapy, discuss target selection, challenges and resolutions of TCR-T technology, combination of TCR-T with other therapies for cancer treatment.
Evolution of ACT
In 1955, Mitchison conferred that adoptive transfer lymph node cells could retain tumor immunity in mouse (13). Almost 30 years later, Rosenberg group demonstrated that LAK cells inhibited the growth of established melanoma using murine B16 metastasis model (3). LAK cells have no restriction on major histocompatibility complex (MHC) for the cytotoxic effect and a broad anticancer spectrum, but have challenges like restrained cell expansion, low efficacy and severe adverse effects. However, the group found that combination of LAK cells and interleukin-2 (IL-2) therapy had a great therapeutic effect on metastatic cancers including renal carcinoma and melanoma (14).
In the late 1980s, Rosenberg group developed TILs as a new kind of ACT by isolating lymphocytes from tumor tissues, and demonstrated its high anti-tumor activities after in vitro expansion and infusion of the cells (4). TIL is a mixture containing different kinds of lymphocytic populations, such as NK cells, NK T cells, CD8+ T cells, CD4+ T cells and γ/δ T cells. The promise of early ACT results has promoted further development in the fields.
Peripheral blood mononuclear cells (PBMCs) had become a major source of lymphocytes for the development of ACT immunotherapy. Schmidt-Wolf et al. demonstrated that CIKs, which produced by inducing and expanding PBMCs with anti-CD3 monoclonal antibodies, IFN-γ and IL-2, had potent antitumor activities (5). CD3+CD56+ effector cells, which comprise only 1–5% PBMCs of healthy person, are the major population of CIK (15). CIKs have the advantages of rapid expansion, high efficient killing various tumor cells, non-human leukocyte antigen (non-HLA) restriction and almost no effect on normal bone marrow, thus can be used as an important complement method in tumor therapy. Such advantages have been harnessed for targeting cancer cells in an antigen-specific manner by further modification of cytokine activated killers (6), or we name this sort of ACT as antigen-specific T cell ACT.
The antigen-specific T cell ACT has been developed mainly into two directions, one of them is CAR-T cell therapy. In 1989, Gross group discovered that CAR-Ts targeting natural antigens on the surface of tumor cells were not dependent on the antigen processing and presentation with HLA (7). Through several decades, CAR-T has been refined continually to improve the safety and efficacy. Generally, CAR-T is genetically constructed to express scFv fusions containing T-cell signaling domains, such as a transmembrane domain, a CD3-derived ITAM signaling domain, and a costimulatory signaling domain (such as CD28, OX40 or 4-1BB, etc.) (2,16,17).
TCR-T as another antigen-specific T cell ACT was inspired by studies of Dembic et al. that α and β gene transfection could redirect the T cell specificity to recognize cognate antigens presented by MHC-I in mouse models in late 80s (8,18). In 1999, Clay et al. subsequently described that genes of a melanoma-associated antigen 1 (MART-1) specific αβ TCR were efficiently transferred to human PBL for treating the cognate antigen positive tumors (19). The remarkable capability of TCR-T has also been illustrated by curing a metastatic melanoma patient with NY-ESO-1 specific CD4+ T cells isolated directly from the patient (20). Objective regression was also noticed in two patients treated with TCR-T specific for MART-1 (21), and a recent report on TCR-T clinic trials showed that affinity improved TCR-T delivered 80% clinical responses with advanced myeloma (22,23). However, TCR-T as a therapeutic strategy for cancer management has its own limitations, and will be discussed in detail later in this review.
Selection of antigens for TCR-T therapy
In TCR-T gene therapy, the first step is to select appropriate tumor antigen for TCR targeting. There are three main type tumor antigens: (I) tumor-associated antigens (TAAs) that are present in healthy tissue but overexpressed in cancer cells, (II) cancer testis antigens (CTAs) that are expressed in cancer cells and testis, fetal ovaries, and trophoblasts (24) and (III) tumor-specific antigens (TSAs) that are just expressed in cancer cells, such as neoantigens and antigens produced by a carcinogenic virus, but not in healthy tissue.
To ensure the safety and effectivity of TCR-T treatment, the targeted antigens presented by MHC on the cancer cells should not exist or be very weakly expressed on normal cells. An adequate T-cell epitope is critic for specific TCR isolation and specifically targeting tumor with constructed TCR-T.
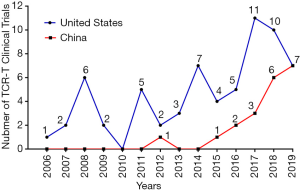
TCR-T therapy has the advantage to recognize most of tumor antigens as they are able to recognize both extracellular and intracellular antigens (16). Over the past decades, there have been many well characterized peptide antigens published for the development of TCR-T. Among these antigen peptides, NY-ESO-1157-165 has been proved to be one of safe and effective targets, so there are more than 30% TCR-T clinical trials targeting NY-ESO-1157-165. HPV-16 E6 and E7 proteins are TSA and closely related with the occurrence of cervical cancer, and TCR-T clinical trials targeting these two antigens have increased rapidly during recent years. In addition, many TAAs, CTAs or TSAs are available for TCR-T therapy, such as MART-1, Wilms’ tumor antigen 1 (WT1), HBsAg, AFP, MAGE (A3, A4, A6, A10), PRAME, CMV, EBV, gag, gp100, HA1, HERV-E, LMP2, CEA or P53 (Figure 1A,B) (25). Figures 2,3 shows the TCR-T clinical trials worldwide until 2019 with data collected from ClinicalTrials.gov.
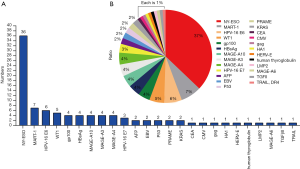

Neoantigens are potential good cancer rejection antigens, which were generated by protein somatic mutation and the resulted peptides are absent from the normal human peptidome. Both tumor-specific DNA alterations and viral open reading frames contribute to the pool of TSA. T cells targeting those antigens are not affected by central T cell tolerance, may effectively control tumor occurrence (26,27).
The limitation of TCR-T and improvement strategies
TCR-T is a kind of TCR gene-modified T cells, and would have some inherited and passive limitations. TCR recognizes HLA restricted antigens, and its binding affinity is selected at the thymus during the T cell maturation, which is a process evolved to prevent the T cells from establishing autoimmunity. However, such self-protection features of immune system can be amplified and utilized by tumor cells to keep from the attacks by T cells. In addition, genetically modified T cells may result in off-target toxicity (28). On the other hand, during the TCR-T treatment, a systemic cytokine release, known as cytokine release syndrome (CRS) (29), may induce a serious adverse event, and uncontrolled CRS will undermine TCR-T applications (22,23). In order to treat patient safely and effectively, researchers and clinicians have developed some strategies to improve the function and safety of TCR-T.
Improving the efficacy
Enhancing TCR affinity
T cells specifically target pHLA with natural affinities selected to ~1–100 µM (30-32), which limit the T cells to recognize tumor cells showing abnormal antigen presentation. Central and peripheral tolerance need low TCR affinity, so there may be no high-affinity CTLs specific for TAA in the peripheral T cell repertoire due to the deletion in the process of thymic selection or tolerance induction. In tumor micro-environment, the downregulation of MHC-peptide complex (pMHC) molecules in most tumors may cause tumor-induced immune tolerance, thus the affinity of TCR for pMHC may limit their function (33,34). Thus, increasing the affinity between TCR and pHLA may overcome tumor-induced immune tolerance and improve the TCR-T function.
Yeast display of TCRs was used to screen stable mutants of the single-chain allo-reactive mouse 2C TCR and to enhance its affinity by 100-fold. The high-affinity 2C TCR endows the transfected T cells with more peptide sensitivity and CD8 independence (35,36). Boulter and his group demonstrated a strategy to generate stable TCR molecules, in which an inter-chain disulfide bond was introduced into the interface between constant domains of the TCR. This method is proved to be suitable for various TCRs (37).
Based on the study of Boulter, the affinity of TCRs were greatly enhanced to pM level using phage display in 2005. Li et al. demonstrated these high-affinity TCRs were specific and sensitive to target cell-surface pHLAs (38). The high-affinity TCR redirected CD8+ T cells released a wider range of soluble factors and more IL-2 than those natural affinity TCR redirected CD8+ T cells. Both HIV wild-type and mutants could be effectively controlled by high-affinity TCR-T cells (39).
Zhao group redirected human PBLs with a series of high-affinity TCRs having KD values of 4 µM, 450 nM, 84 nM, 5 nM and 26 pM to verify if T cells expressing the high-affinity TCRs would be more sensitive to cancer cells. And Robbins group also checked different mutations of 1G4 TCR for their functions and specificity. The results showed, in order to ensure the safety and reduce the cross-reactivity of TCRs to self-peptide antigens, that the affinity of TCRs to pHLA should be intermedium but not too high (40,41).
In 2006, Rosenberg group reported that there were about 12% (2 of 17) tumor regression while using wild-type MART-127-35-specific TCR-T to treat melanoma patients (NCT00091104). The infused cells could be detected in patients for a long time (21). Furthered studies indicated that there were 12% (4 of 34) and 30% (6 of 20) tumor responses while treating patients with low (DMF4) and high (DMF5) affinity TCR specific for the same MART-1 antigen, respectively (NCI-07-C-0174 and NCI-07-C-0175) (42). In addition, about 67% (4 of 6) patients with synovial cell sarcomas and 45% (5 of 11) patients with melanoma showed objective clinical responses while treated by an engineered high-avidity NY-ESO-1 TCR. And about 18% (2 of 11) patients with melanoma exhibited absolute tumor regressions which persisted for 1 year (NCI-08-C-0121) (43,44). In the clinic trials of affinity-enhanced NY-ESOC259-specific TCR-T, 16 of 20 patients (80%) with advanced multiple myeloma showed encouraging clinical responses, with 19.1 months of median progression-free survival (NCT01352286) (22). It suggested that tumor rejection may be improved with the increasement of TCR affinity.
Increase TCR expression
The response thresholds were substantially lowered and the T cell population decayed gradually if TCR levels were reduced (45). Human TCR assembled with murine constant regions could express more exogenous TCR and mediate higher levels of cytokine secretion (46). Moreover, mutation of the amino acid on specific sites of α chain and β chain to cysteine to introduce an additional disulfide bond, suppression of the endogenous TCR expression by siRNA or zinc finger nuclease (ZFN), linking TCRα and β chain gene fragments with self-splicing polypeptide 2A (2A), could reduce the mismatch and increase the expression of exogenous TCR (47-50).
In this process of immunological synapse, the activation of T cells is triggered by the combination of TCR and pMHC and then mediated by CD3 molecule. Co-transfection of TCR and CD3 enhanced the expression of TCR and led to more rapid tumor clearance (51).
Cytokines and co-stimulatory signals
The family cytokines of common cytokine-receptor γ-chain (γc), containing IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21, make an important impact in the development, proliferation, differentiation and survival of T cells (52). Providing exogenous IL-2 enhances the proliferation and survival of T cells during the death phase (53). In addition, IL-7 has effect in the downregulation of Socs3 levels and the promotion of IL-6 production, thus to promote immune response to chronic infection (54). IL-15 and IL-21 can enhance the cytotoxicity of gp100/HLA-A2-directed TCR-T, as those cytokines have the ability to enhance the expression of granzymes A and B, and perforin 1 (55). T-cells specific for WT1 pretreated with IL-21 could develop memory cells and extend life span of all four patients (56). Tyrosinase-specific TCR-T conditioned with IL-12 exhibited significantly enhanced functional activity, as granzyme B expression was heightened and peptide-specific CD107a degranulation was elevated (57). White blood cells secreting IL-2/IL-12 and recognizing NY-ESO-1 antigen were used to cure metastatic cancer (NCT01457131). During T cells culture, the adjunction of cytokines has a significant impact on the subsequent therapy in vivo.
A costimulatory signal, such as a second stimulatory signal provided by CD28/B7, is also important for T-cell activation. In vitro experiments indicated that EBV-specific TCR-T co-expressed with CD28 can dramatically enhance antigen-specific IFN-γ production (58). In vivo mouse experiments showed that TCR and CD3 co-transducted T-cells infiltrated tumors faster and accumulated in larger numbers, and resulted in more rapid tumor clearance compared with T cells redirected by TCR only (51).
Improving the safety
Insert suicide genes
CRS frequently occurred as serious side effect and potentially led to the fatal complication in the TCR-T therapy. TCR-T may cause on-target toxicity as healthy tissues may display the targeted antigen (59). It is not easy to predict the specificity of the mis-paired TCR combinations, but the non-specificity may lead to off-target toxicity that can cause major damage to normal organization (60). The insertion of the suicide genes is an alternative strategy of optimizing safety and TCR-T cell regulation in vivo in case of heavy CRS or toxicity. The herpes simplex virus thymidine kinase (HSV-TK) is the largely tested suicide gene in humans and is documented to be safe and effective, which confers lethal sensitivity of TCR-T to ganciclovir (61,62). Other suicide genes, such as a truncated human epidermal growth factor receptor (tEGFR), the small molecule dimerizer, the inducible caspase 9 safety switch (iCasp9), an oligopeptide, are also frequently used to improve the safety (63,64).
Rigorous assessment the potential co-expression of peptides in normal tissues
To prevent the on-target toxicity in TCR-T immunotherapy, it needs to examine and confirm whether the targeted antigen could be co-expressed in normal tissues or organs. For instance, in an AFP specific TCR-T therapy, the alanine scan (X-scan), different kinds of tissues, cell types and HLA alleles were applied to test the specificity and safety (65).
Knockdown of endogenous TCRs
The knockdown of endogenous TCRs may reduce the mispairing and increase exogenous redirected TCR expression, as co-expressed of exogenous and endogenous TCR chains on T cells may lead to competition for surface expression and inappropriate matching. Nucleases genome editing, RNA interfere (RNAi), Zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALEN) and CRISPR/Cas9 could be used to knockdown that gene (66,67).
Can engineering neoantigen-reactive T cells cover both efficacy and safety?
The repertoires of TCR and antigen are large, but we only found a small part for the development of TCR-T to carry out clinical trials. Meanwhile, as TSAs (including neoantigens) don’t exist in the normal organs, TCR-T therapy focusing on these targets may have the best safety feature. The number of TAAs and CTAs targets is limited, thus targeting neo-antigens would be advantageous for the development of TCR-T immunotherapy (27). Cancer-specific mutations maybe possible to be detected by next-generation sequencing technology in individual patients (68-70). TCR-T targeting neo-antigens such as KRAS, has also been tested in clinical trials (NCT03190941 and NCT03745326), and will be realistic and effective to treat tumor patients in the near future.
TCR-T combination with other therapies
Surgical resection and RT make significant effect on controlling tumor growth, but are often difficult to eradicate metastases or residual cancer cells. Chemotherapy could inhibit rapidly growing tumor cells, but the side effects and drug resistance limits its efficacy to a certain extent. Immunological checkpoint antibodies have low tumor response rates, nearly 20–30% at most of the time. Combining ACT with other therapeutic methods will be likely to provide long-lasting benefits in clinical therapy. Below we will summarize the combination of TCR-T with resection, RT, chemotherapy, immune cells and immune checkpoint. Table 1 shows the TCR-T therapy clinical trials combined with other therapies, with data collected from ClinicalTrials.gov.
Table 1
| Antigen | Disease | Combination | ClinicalTrials.gov identifier | Location | Year |
|---|---|---|---|---|---|
| MART-1 | Melanoma (skin) | Peptide vaccine and aldesleukin | NCT00091104 | United States | 2004 |
| gp100 | Melanoma (skin) | gp100-fowlpox vaccine | NCT00085462 | United States | 2004 |
| p53 | Kidney cancer melanoma (skin) unspecified adult solid tumor, protocol specific | Dendritic cell vaccination | NCT00704938 | United States | 2008 |
| MART-1 | Metastatic melanoma skin cancer | ALVAC MART-1 vaccine | NCT00612222 | United States | 2008 |
| gp100 | Metastatic melanoma skin cancer | ALVAC gp100 vaccine | NCT00610311 | United States | 2008 |
| MART-1; gp100 | Melanoma skin cancer | Peptide vaccines | NCT00923195 | United States | 2009 |
| NY-ESO-1 | Metastatic cancer metastatic melanoma metastatic renal cancer | Secrete IL-2 | NCT01457131 | United States | 2011 |
| NY-ESO-1 | Malignant neoplasm | Dendritic cells and IL-2 | NCT01697527 | United States | 2012 |
| NY-ESO-1 | Unspecified adult solid tumor, protocol specific | CTLA-4 antibody blockade | NCT02070406 | United States | 2014 |
| NY-ESO-1 | Adult solid neoplasm childhood solid neoplasm metastatic neoplasm | Nivolumab PD-1 blockade | NCT02775292 | United States | 2016 |
| NY-ESO-1; mTCR | Metastatic malignant neoplasm, metastatic malignant neoplasm in the brain | cyclophosphamide and fludarabine phosphate | NCT02774291 | United States | 2016 |
| NY-ESO-1 | Ovarian, primary peritoneal, or fallopian tube cancer | Decitabine | NCT03017131 | United States | 2017 |
| NY-ESO-1 | Multiple myeloma | Anti-PD-1 antibody pembrolizumab | NCT03168438 | United Kingdom | 2017 |
| HPV16+ E6 | NHSCC or cervical cancer | Anti-PD-1 auto-secreted | NCT03578406 | China | 2018 |
| NY-ESO-1 | Multiple myeloma; melanoma, synovial sarcoma, myxoid/round cell liposarcoma | CRISPR gene edited to eliminate endogenous TCR and PD-1 | NCT03399448 | United States | 2018 |
| NY-ESO-1 | Neoplasms | Anti-PD-1 antibody pembrolizumab | NCT03697824 | United Kingdom | 2018 |
| EBV | Head and neck squamous cell carcinoma | Anti-PD-1 auto-secreted | NCT04139057 | China | 2019 |
| – | HIV/AIDS | Chidamide | NCT03980691 | China | 2019 |
| – | Solid tumor | Anti-PD-1 | NCT03970382 | United States | 2019 |
TCR-T, T-cell receptor engineered T cell therapy; MART-1, melanoma-associated antigen 1; IL-2, interleukin-2.
Open surgery therapy is accompanied by the problem of low survival rate and tumor recurrence. The combination of surgical resection with TCR-T seems to be synergistic in eliminating residual cancer cells and preventing tumor recurrence. For instance, the team of Antonio Bertoletti used HBs-specific TCR-T to eradicate residual HCC cells in the blood after the patient who had undergone liver transplantation, which could provide valuable prophylaxis against relapse (NCT02686372, NCT02719782, NCT03634683) (71-73). Meanwhile, tumor tissues and TILs after surgical resections can also be processed for immunotherapies.
It suggests that RT can reduce the local and distal diseases by regulating TM and changing the tumor cell intrinsic characteristics (74). While chemotherapy can directly eradicate tumor cells by direct cytotoxicity and immune-mediated cytotoxicity. In the clinical research (NCT00923195), patients were treated with 5 days of chemotherapy and 2 days of RT, so that the immune system was ready for receiving the MART-1 and gp100 specific TCR-T cells. The combination between chemotherapy/radiation and TCR-T led to the development of more efficacious ACT treatments for cancer (75).
Peptide vaccines and DC were also tried to be used together with TCR-T (NCT00704938, NCT00923195). In the clinical of NCT00923195, the procedure also used the anti-MART-126-35(27L) peptide vaccines or the anti-gp100154-162 peptide vaccines to stimulate immune cells, in order to increase the function of MART-1 and gp100 specific TCR-T cells (75). The combination therapy made good effect on modulating DC maturation, promoting cancer immunogenicity, increasing tumor antigen presentation, and motivating immune responses.
Two important checkpoint molecules CTLA-4 and PD-1 are major negative costimulatory molecules, which can inhibit the function of T-cell in TM (76). To overcome the immune suppression, T cells can be combined with anti-CTLA-4 and/or anti-PD-1 antibodies to block the immunosuppressive signaling pathway.
FDA has approved the CTLA-4 antibody, Ipilimumab, to treat patients with advanced melanoma, and 15–20% of treated subjects could achieve lasting benefits up to 2.5 years. Meanwhile, the PD-1 monoclonal antibodies pembrolizumab and nivolumab have also been approved to treat advanced melanoma (77). Recently, TCR-T clinical therapies combined with CTLA-4 antibody and PD-1 antibody have also been conducted to promote the function of T cells and break immune tolerance (NCT02070406, NCT02775292, NCT03168438, NCT03578406, NCT03697824, NCT04139057).
Conclusions
TCR-T is a passive immunotherapy of cancer. To prepare for therapeutic injection of TCR-T, a large number of T cells can be efficiently grown in vitro by manual or machine, which could spare the lymphocytes from the influence of immunosuppression environment in vivo. Various host preconditioning regimens can also be applied before TCR-T to create an environment favoring infused T cell to grow. These important advantages make TCR-T as a promising cancer immunotherapy (78,79).
Ken Garber reported that TCR-T have an advantage over CAR-T to treat solid tumors, though the field is still under study (16). These encouraging clinical results on solid tumor, such as metastatic melanoma demonstrated the function of TCR-T to treat cancer. TCR-T may cause on-target toxicity if the targeted antigens, mainly TAA, not only express on tumor cells but also express on normal tissues, even at a lower level. Therefore, targeting antigens expressed on cancers, such as virus specific antigen and neoantigen, have been demonstrated as a reasonable strategy to get off the beaten path to reduce the collateral damage.
TCR-T is promising to be a remarkably effective immunotherapy for patients with cancer. On the other hand, it is important to further the study of those issues, such as how to predict and control adverse events, how to search for new targets including neo-antigens, how to efficiently produce T cells at low cost, and how to combine TCR-T with other antitumor therapies. We imagine that TCR-T adoptive immunotherapy will treat patients with many malignancies effectively and safely by resolving these issues in the near future, especially in the solid tumor treatment.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2020.02.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Yang F, Jin H, Wang J, et al. Adoptive cellular therapy (ACT) for cancer treatment. Adv Exp Med Biol 2016;909:169-239. [Crossref] [PubMed]
- Mazumder A, Rosenberg SA. Successful immunotherapy of natural killer-resistant established pulmonary melanoma metastases by the intravenous adoptive transfer of syngeneic lymphocytes activated in vitro by interleukin 2. J Exp Med 1984;159:495-507. [Crossref] [PubMed]
- Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 1986;233:1318-21. [Crossref] [PubMed]
- Schmidt-Wolf IG, Negrin RS, Kiem HP, et al. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med 1991;174:139-49. [Crossref] [PubMed]
- Kang S, Li Y, Bao Y, et al. High-affinity T cell receptors redirect cytokine-activated T cells (CAT) to kill cancer cells. Front Med 2019;13:69-82. [Crossref] [PubMed]
- Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A 1989;86:10024-8. [Crossref] [PubMed]
- Dembić Z, Haas W, Weiss S, et al. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature 1986;320:232-8. [Crossref] [PubMed]
- Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509-18. [Crossref] [PubMed]
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507-17. [Crossref] [PubMed]
- Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther 2017;25:285-95. [Crossref] [PubMed]
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531-44. [Crossref] [PubMed]
- Mitchison NA. Studies on the immunological response to foreign tumor transplants in the mouse. I. The role of lymph node cells in conferring immunity by adoptive transfer. J Exp Med 1955;102:157-77. [Crossref] [PubMed]
- Rosenberg SA, Lotze MT, Muul LM, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 1985;313:1485-92. [Crossref] [PubMed]
- Schmidt-Wolf IG, Lefterova P, Mehta BA, et al. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol 1993;21:1673-9. [PubMed]
- Garber K. Driving T-cell immunotherapy to solid tumors. Nat Biotechnol 2018;36:215-9. [Crossref] [PubMed]
- Charrot S, Hallam S. CAR-T cells: future perspectives. Hemasphere 2019;3:e188 [Crossref] [PubMed]
- Dembić Z, Haas W, Zamoyska R, et al. Transfection of the CD8 gene enhances T-cell recognition. Nature 1987;326:510-1. [Crossref] [PubMed]
- Clay TM, Custer MC, Sachs J, et al. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol 1999;163:507-13. [PubMed]
- Hunder NN, Wallen H, Cao J, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med 2008;358:2698-703. [Crossref] [PubMed]
- Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006;314:126-9. [Crossref] [PubMed]
- Rapoport AP, Stadtmauer EA, Binder-Scholl GK, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med 2015;21:914-21. [Crossref] [PubMed]
- D'Angelo SP, Melchiori L, Merchant MS, et al. Antitumor activity associated with prolonged persistence of adoptively transferred NY-ESO-1 (c259)T cells in synovial sarcoma. Cancer Discov 2018;8:944-57. [Crossref] [PubMed]
- Simpson AJ, Caballero OL, Jungbluth A, et al. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 2005;5:615-25. [Crossref] [PubMed]
- Mo Z, Du P, Wang G, et al. The multi-purpose tool of tumor immunotherapy: gene-engineered T cells. J Cancer 2017;8:1690-703. [Crossref] [PubMed]
- Gilboa E. The makings of a tumor rejection antigen. Immunity 1999;11:263-70. [Crossref] [PubMed]
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348:69-74. [Crossref] [PubMed]
- Linette GP, Stadtmauer EA, Maus MV, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013;122:863-71. [Crossref] [PubMed]
- Xu XJ, Tang YM. Cytokine release syndrome in cancer immunotherapy with chimeric antigen receptor engineered T cells. Cancer letters 2014;343:172-8. [Crossref] [PubMed]
- Ashton-Rickardt PG, Bandeira A, Delaney JR, et al. Evidence for a differential avidity model of T cell selection in the thymus. Cell 1994;76:651-63. [Crossref] [PubMed]
- Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature 1999;402:255-62. [Crossref] [PubMed]
- van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annual review of immunology 2003;21:659-84. [Crossref] [PubMed]
- Manz BN, Jackson BL, Petit RS, et al. T-cell triggering thresholds are modulated by the number of antigen within individual T-cell receptor clusters. Proc Natl Acad Sci U S A 2011;108:9089-94. [Crossref] [PubMed]
- Dustin ML, Depoil D. New insights into the T cell synapse from single molecule techniques. Nat Rev Immunol 2011;11:672-84. [Crossref] [PubMed]
- Holler PD, Lim AR, Cho BK, et al. CD8(-) T cell transfectants that express a high affinity T cell receptor exhibit enhanced peptide-dependent activation. J Exp Med 2001;194:1043-52. [Crossref] [PubMed]
- Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity 2003;18:255-64. [Crossref] [PubMed]
- Boulter JM, Glick M, Todorov PT, et al. Stable, soluble T-cell receptor molecules for crystallization and therapeutics. Protein Eng 2003;16:707-11. [Crossref] [PubMed]
- Li Y, Moysey R, Molloy PE, et al. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol 2005;23:349-54. [Crossref] [PubMed]
- Varela-Rohena A, Molloy PE, Dunn SM, et al. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nature medicine 2008;14:1390-5. [Crossref] [PubMed]
- Zhao Y, Bennett AD, Zheng Z, et al. High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. The Journal of Immunology 2007;179:5845-54. [Crossref] [PubMed]
- Robbins PF, Li YF, El-Gamil M, et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol 2008;180:6116-31. [Crossref] [PubMed]
- Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 2009;114:535-46. [Crossref] [PubMed]
- Robbins PF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011;29:917-24. [Crossref] [PubMed]
- Robbins PF, Kassim SH, Tran TL, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res 2015;21:1019-27. [Crossref] [PubMed]
- Labrecque N, Whitfield LS, Obst R, et al. How much TCR does a T cell need? Immunity 2001;15:71-82. [Crossref] [PubMed]
- Cohen CJ, Zhao YB, Zheng ZL, et al. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Research 2006;66:8878-86. [Crossref] [PubMed]
- Cohen CJ, Li YF, El-Gamil M, et al. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Research 2007;67:3898-903. [Crossref] [PubMed]
- Leisegang M, Engels B, Meyerhuber P, et al. Enhanced functionality of T cell receptor-redirected T cells is defined by the transgene cassette. J Mol Med (Berl) 2008;86:573-83. [Crossref] [PubMed]
- Okamoto S, Mineno J, Ikeda H, et al. Improved expression and reactivity of transduced tumor-specific TCRs in human lymphocytes by specific silencing of endogenous TCR. Cancer Research 2009;69:9003-11. [Crossref] [PubMed]
- Provasi E, Genovese P, Lombardo A, et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat Med 2012;18:807-15. [Crossref] [PubMed]
- Ahmadi M, King JW, Xue SA, et al. CD3 limits the efficacy of TCR gene therapy in vivo. Blood 2011;118:3528-37. [Crossref] [PubMed]
- Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol 2009;9:480-90. [Crossref] [PubMed]
- Blattman JN, Grayson JM, Wherry EJ, et al. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nature medicine 2003;9:540-7. [Crossref] [PubMed]
- Pellegrini M, Calzascia T, Toe JG, et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell 2011;144:601-13. [Crossref] [PubMed]
- Pouw N, Treffers-Westerlaken E, Kraan J, et al. Combination of IL-21 and IL-15 enhances tumour-specific cytotoxicity and cytokine production of TCR-transduced primary T cells. Cancer Immunol Immunother 2010;59:921-31. [Crossref] [PubMed]
- Chapuis AG, Ragnarsson GB, Nguyen HN, et al. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci Transl Med 2013;5:174ra27 [Crossref] [PubMed]
- Rubinstein MP, Su EW, Suriano S, et al. Interleukin-12 enhances the function and anti-tumor activity in murine and human CD8(+) T cells. Cancer Immunol Immunother 2015;64:539-49. [Crossref] [PubMed]
- Schaft N, Lankiewicz B, Drexhage J, et al. T cell re-targeting to EBV antigens following TCR gene transfer: CD28-containing receptors mediate enhanced antigen-specific IFNgamma production. Int Immunol 2006;18:591-601. [Crossref] [PubMed]
- Stauss HJ, Morris EC. Immunotherapy with gene-modified T cells: limiting side effects provides new challenges. Gene Ther 2013;20:1029-32. [Crossref] [PubMed]
- Bendle GM, Linnemann C, Hooijkaas AI, et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med 2010;16:565-70, 1p following 570.
- Greco R, Oliveira G, Stanghellini MT, et al. Improving the safety of cell therapy with the TK-suicide gene. Front Pharmacol 2015;6:95. [Crossref] [PubMed]
- Lupo-Stanghellini MT, Provasi E, Bondanza A, et al. Clinical impact of suicide gene therapy in allogeneic hematopoietic stem cell transplantation. Hum Gene Ther 2010;21:241-50. [Crossref] [PubMed]
- Straathof KC, Pule MA, Yotnda P, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood 2005;105:4247-54. [Crossref] [PubMed]
- Liu L, Sommermeyer D, Cabanov A, et al. Inclusion of Strep-tag II in design of antigen receptors for T-cell immunotherapy. Nat Biotechnol 2016;34:430-4. [Crossref] [PubMed]
- Docta RY, Ferronha T, Sanderson JP, et al. Tuning T-cell receptor affinity to optimize clinical risk-benefit when targeting alpha-fetoprotein-positive liver cancer. Hepatology 2019;69:2061-75. [Crossref] [PubMed]
- Bunse M, Bendle GM, Linnemann C, et al. RNAi-mediated TCR knockdown prevents autoimmunity in mice caused by mixed TCR dimers following TCR gene transfer. Molecular Therapy 2014;22:1983-91. [Crossref] [PubMed]
- Osborn MJ, Webber BR, Knipping F, et al. Evaluation of TCR gene editing achieved by TALENs, CRISPR/Cas9, and megaTAL nucleases. Molecular Therapy 2016;24:570-81. [Crossref] [PubMed]
- Verdegaal EM, de Miranda NF, Visser M, et al. Neoantigen landscape dynamics during human melanoma-T cell interactions. Nature 2016;536:91-5. [Crossref] [PubMed]
- Klebanoff CA, Rosenberg SA, Restifo NP. Prospects for gene-engineered T cell immunotherapy for solid cancers. Nature medicine 2016;22:26-36. [Crossref] [PubMed]
- Linnemann C, Heemskerk B, Kvistborg P, et al. High-throughput identification of antigen-specific TCRs by TCR gene capture. Nat Med 2013;19:1534-41. [Crossref] [PubMed]
- Qasim W, Brunetto M, Gehring AJ, et al. Immunotherapy of HCC metastases with autologous T cell receptor redirected T cells, targeting HBsAg in a liver transplant patient. J Hepatol 2015;62:486-91. [Crossref] [PubMed]
- Koh S, Tan AT, Li L, et al. Targeted therapy of hepatitis B virus-related hepatocellular carcinoma: present and future. Diseases 2016; [Crossref] [PubMed]
- Tan AT, Yang N, Lee Krishnamoorthy T, et al. Use of expression profiles of HBV-DNA integrated into genomes of hepatocellular carcinoma cells to select T cells for immunotherapy. Gastroenterology 2019;156:1862-76.e9. [Crossref] [PubMed]
- de la Cruz-Merino L, Illescas-Vacas A, Grueso-López A, et al. Radiation for awakening the dormant immune system, a promising challenge to be explored. Front Immunol 2014;5:102. [Crossref] [PubMed]
- Abate-Daga D, Hanada K, Davis JL, et al. Expression profiling of TCR-engineered T cells demonstrates overexpression of multiple inhibitory receptors in persisting lymphocytes. Blood 2013;122:1399-410. [Crossref] [PubMed]
- Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 2013;13:227-42. [Crossref] [PubMed]
- Metcalfe W, Anderson J, Trinh VA, et al. Anti-programmed cell death-1 (PD-1) monoclonal antibodies in treating advanced melanoma. Discovery Medicine 2015;19:393-401. [PubMed]
- Galluzzi L, Vacchelli E, Bravo-San Pedro JM, et al. Classification of current anticancer immunotherapies. Oncotarget 2014;5:12472-508. [Crossref] [PubMed]
- Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348:62-8. [Crossref] [PubMed]
Cite this article as: Liu Q, Cai W, Zhang W, Li Y. Cancer immunotherapy using T-cell receptor engineered T cell. Ann Blood 2020;5:5.


