The multifactorial roles of IL-34 in immune responses
Introduction
Interleukin 34 (IL-34) is a newly discovered cytokine that regulates the survival, differentiation and function of macrophage, osteoclast as well as microglia. Like other cytokines (1-15), it exerts its roles in target cells depending upon its receptor distribution. Although several receptors of IL-34 have been identified, the colony-stimulating factor 1 receptor (CSF1R, also called CD115) is a major one. However, CSF1R also binds with its archetypical ligand colony stimulating factor 1 (CSF-1), or called macrophage colony stimulating factor (M-CSF), for mediating mononuclear phagocyte, especially participates in the development of macrophage, dendritic cell, Langerhans and microglia (16). In CSF1-KO mice, Langerhans and microglia are unaffected, but they are absent in CSF1R-deficient mice. It suggests CSF1 is a dispensable cytokine for the development of Langerhans and microglia, and IL-34, alternative ligand of CSF1R, is nonredundant for differentiation of myeloid progenitor cell in the skin and central nervous system (CNS) (17). In 2009, Wei et al. (18) revealed IL-34, like CSF1, phosphorylates the membrane receptor CSF1R tyrosine phosphorylation, enhances the proliferation and viability in CSF1R+ macrophage via activating intracellular signaling pathway MAPK and ERK1/2. Additionally, IL-34 was identified as osteoclastogenic cytokine product by multiple myeloma cells that promote osteoclast formation and deteriorate osteolytic disease in multiple myeloma (19). Indeed, the osteocloast formation usually needs RANKL and M-CSF (20,21). Thus, IL-34 can directly initiate macrophage and monocyte responses as a pro-inflammatory agent by combining its receptor CSF-1. Nonetheless, IL-34 can be produced by regulatory cells (22) and then plays a role in suppressing immune responses. It is clear now that Treg cells are a crucial negative player that regulates immune balance and prevention of autoimmune and inflammatory diseases (23-31).
Source of IL-34
It is well known that IL-34 is a cytokine mainly produced by macrophage, monocyte, and microglia (32). Additionally, it is also produced by synovial fibroblasts in rheumatoid arthritis (RA) and hepatocytes in hepatitis virus infection. As synovial fibroblast plays a critical role in the pathogenesis of rheumatoid arthritis (33-37), this is likely that IL-34 serves as an inflammatory cytokine to participate in inflammation. In addition, IL-34 has also been found to be expressed in regulatory T cell (Treg) and IL34+ Treg has stronger immunoregulatory capacity (22). Thus, IL-34 could be a double sword that plays a different role under the distinct conditions and environments.
IL-34 in infection immunity
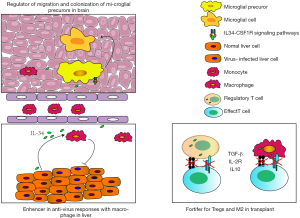
IL-34 has been recognized as an anti-virus cytokine during infection with influenza A virus (IAV) (38), hepatitis B virus (HBV) (39,40), hepatitis C virus (HCV) (41), human immunodeficiency virus (HIV) (42), that is produced in response to virus infection by inhibiting virus replication accompanied infiltration of macrophage in peripheral tissue or microglia in CNS. Virus infection is a critical cause of immune responses, and interleukins are secreted during the host’s anti-virus process that plays a different role in different tissues depending upon the types of virus-infection. For example, IL-22 is one of IL-10 family members to play a role in inhibiting inflammatory responses, was enhanced in IAV infected patients to induce IL-34 production that feedbacks to regulate IL-22 expression (38). In HCV-infection, IL-34 from hepatocytes around liver lesion can drive the existing macrophages to secret chemokine ligands (CCLs) and chemokine receptors (CCRs) to recruit monocytes and macrophages, to release platelet-derived growth factor (PDGF), and transforming growth factor beta (TGF-β) that induce liver fibrosis (41). The detailed descriptions of the anti-virus immune responses of IL-34 are summarized in Table 1 and Figure 1.
Table 1
| Virus | Producer | Target cell | Role of IL-34 | Synergistic molecule | Effect | References |
|---|---|---|---|---|---|---|
| IAV | IAV-infected cells in PBMC | Th17 | Feedback induce IL-22 expression | IL-22 | ↑IL-22; drive inflammatory | ( |
| HBV | Normal hepatocyte | Hepatoma cell | Inhibit HBV expression | Unclear | ↑Liver fibrosis | ( |
| HCV | Hepatocytes around liver lesion | Monocyte; macrophage | Recruit monocyte/macrophage | ↓ | ↓Collagenase; ↑collagen synthesis; ↓MMP1 | ( |
| HIV | Microglia | Peripheral macrophages | Recruit peripheral macrophages into brain | Receptor-type protein-tyrosine phosphatase zeta (RTPTP-ζ) | Drive brain reconstitution peripheral macrophages into microglial-like cells | ( |
Taken together, role IL-34 exerts in the infection remains to be further studied, existing researches focus only on viral infections but not on bacterial, fungal, and parasitic infection. These studies demonstrate that IL-34 requires the responses of monocyte and macrophage in inflammation initiated by the virus to exert its function, but the triggering mechanism of initial IL-34 expression is still elusive. Interestingly, A contradictory phenomenon was reported that IL-34 is enhanced in IAV-infection while decreased in HBV-infection (38,40). Alternatively, IL-34 functional differences may be related to the timing and specific tissue localization.
IL-34 in autoimmune diseases
Autoimmune diseases are a systemic disease characterized by excessive immune responses involving many immune tissues, cells and interleukins (43-47). The high serum level of IL-34 was observed in many autoimmune diseases (48-53). Indeed, the involvement of IL-34 has been assessed in the initiation and development of autoimmune inflammation. Many correlation-studies assessed the serum level of IL-34 and suggested IL-34 is a positive biomarker for autoimmune response. Osteoclasts are the potent drivers and effector that participated in both inflammatory and erosion of bone and cartilage in rheumatoid arthritis (RA). The serum level of IL-34 was higher in RA patients than osteoarthritis patients, more than healthy control, and was positively accompanied with a higher radiographic progression and rheumatoid factors expression (54-56). The maturation of osteoclastogenesis is successfully induced by the supernatants of tumor necrosis factor-α (TNF-α) stimulated periodontal ligament cells added to the differentiation medium of human peripheral blood monocytes/macrophage cells with the receptor activator of nuclear factor kappa-B ligand (RANKL) in vitro, but the number of osteoclast was decreased after the anti-IL-34 IgG was added to the cultures (57). Obviously, the high expression of IL-34 in RA patients is inseparable from its ability to promote the differentiation of monocytes, especially osteoclasts, and thus participates in the development of RA.
High serum level of IL-34 was also observed in patients with systemic lupus erythematosus (SLE), and the worse the clinical features and the higher IL-34 expression (48,58). The SLE is a systemic autoimmune disease characterized by a large number of activated autoreactive T, B cells and followed by immune dysfunction (59-63). However, abnormal secretion of many cytokines can lead to immune dysfunction, but how the IL34 is involved in the course of SLE remains to be unclear and deserves a deep study in the future.
IL-34 in transplant immunity
Induction of transplant tolerance provides an efficiently therapy in both the acute rejection and the chronic allograft dysfunction (64-66). The therapy of anti-graft immunity has benefited from the development of Tregs and immune-suppressive monocytes in both experimental allograft-models and transplantation-patients (67,68). Yet, it is still unclear how IL-34 regulates Tregs and suppressive monocytes and how the balance of pro-inflammatory and anti-inflammatory role of IL-34 is regulated. Recently, IL-34 was demonstrated as a key mediator as it was highly expressed in CD8+CD45RClo Tregs and regulatory macrophages (69,70). In fact, Treg cells and M2 macrophage have an outstanding anti-inflammation role in organ transplantation and other diseases (71-77). Bezie and colleagues has found that CD8+CD45RClo Tregs expressed a high level of IL-34. IL-34-treated macrophages when co-cultured with allogeneic PBMCs, significantly increased the percentage and number of Foxp3+CD8+CD45RClo and Foxp3+CD4+CD45RClo Tregs. Importantly, the IL-34-expanded Foxp3+CD8+CD45RClo Tregs showed an enhanced immunosuppressive capability (22), as shown in Figure 1. Moreover, long-term transplant survival was successfully established in rat cardiac allograft model by IL-34 overexpression to expand Foxp3+CD8+CD45RClo Tregs that promotes macrophage to easily migrate into graft, then exhausts the autoreactive effect T cells and inhibits the production of autoantibody.
Concluding remarks
IL-34 is functionally similar to CSF-1, playing a key role in development and function of mononuclear linage cells. However, its effect is strongly determined by timing and sources. It is yet unclear what is its exact role in the diseases and patients. The further study is strongly suggested.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2019.12.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lai X, Li X, Chang L, et al. IL-19 Up-Regulates Mucin 5AC Production in Patients With Chronic Rhinosinusitis via STAT3 Pathway. Front Immunol 2019;10:1682. [Crossref] [PubMed]
- Ryffel B, Huang F, Robinet P, et al. Blockade of IL-33R/ST2 Signaling Attenuates Toxoplasma gondii Ileitis Depending on IL-22 Expression. Front Immunol 2019;10:702. [Crossref] [PubMed]
- Deng YN, Bellanti JA, Zheng SG. Essential Kinases and Transcriptional Regulators and Their Roles in Autoimmunity. Biomolecules 2019; [Crossref] [PubMed]
- Chen Y, Colello J, Jarjour W, et al. Cellular Metabolic Regulation in the Differentiation and Function of Regulatory T Cells. Cells 2019; [Crossref] [PubMed]
- Yang S, Xie C, Chen Y, et al. Differential roles of TNFalpha-TNFR1 and TNFalpha-TNFR2 in the differentiation and function of CD4(+)Foxp3(+) induced Treg cells in vitro and in vivo periphery in autoimmune diseases. Cell Death Dis 2019;10:27. [Crossref] [PubMed]
- Mao YM, Zhao CN, Leng J, et al. Interleukin-13: A promising therapeutic target for autoimmune disease. Cytokine Growth Factor Rev 2019;45:9-23. [Crossref] [PubMed]
- Yang S, Wang J, Brand DD, et al. Role of TNF-TNF Receptor 2 Signal in Regulatory T Cells and Its Therapeutic Implications. Front Immunol 2018;9:784. [Crossref] [PubMed]
- Guan SY, Leng RX, Tao JH, et al. Hypoxia-inducible factor-1alpha: a promising therapeutic target for autoimmune diseases. Expert Opin Ther Targets 2017;21:715-23. [Crossref] [PubMed]
- Luo Y, Zheng SG. Hall of Fame among Pro-inflammatory Cytokines: Interleukin-6 Gene and Its Transcriptional Regulation Mechanisms. Front Immunol 2016;7:604. [Crossref] [PubMed]
- Madouri F, Guillou N, Fauconnier L, et al. Caspase-1 activation by NLRP3 inflammasome dampens IL-33-dependent house dust mite-induced allergic lung inflammation. J Mol Cell Biol 2015;7:351-65. [Crossref] [PubMed]
- Su W, Wan Q, Huang J, et al. Culture medium from TNF-alpha-stimulated mesenchymal stem cells attenuates allergic conjunctivitis through multiple antiallergic mechanisms. J Allergy Clin Immunol 2015;136:423-32.e8. [Crossref] [PubMed]
- Pan HF, Leng RX, Li XP, et al. Targeting T-helper 9 cells and interleukin-9 in autoimmune diseases. Cytokine Growth Factor Rev 2013;24:515-22. [Crossref] [PubMed]
- Chen M, Lin X, Liu Y, et al. The function of BAFF on T helper cells in autoimmunity. Cytokine Growth Factor Rev 2014;25:301-5. [Crossref] [PubMed]
- Tan Z, Jiang R, Wang X, et al. RORgammat+IL-17+ neutrophils play a critical role in hepatic ischemia-reperfusion injury. J Mol Cell Biol 2013;5:143-6. [Crossref] [PubMed]
- Yang X, Zheng SG. Interleukin-22: a likely target for treatment of autoimmune diseases. Autoimmun Rev 2014;13:615-20. [Crossref] [PubMed]
- Lin H, Lee E, Hestir K, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science 2008;320:807-11. [Crossref] [PubMed]
- Wang Y, Szretter KJ, Vermi W, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol 2012;13:753-60. [Crossref] [PubMed]
- Wei S, Nandi S, Chitu V, et al. Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. Journal of Leukocyte Biology 2010;88:495-505. [Crossref] [PubMed]
- Baghdadi M, Ishikawa K, Nakanishi S, et al. A role for IL-34 in osteolytic disease of multiple myeloma. Blood Advances 2019;3:541-51. [Crossref] [PubMed]
- Luo Y, Wu W, Gu J, et al. Human gingival tissue-derived MSC suppress osteoclastogenesis and bone erosion via CD39-adenosine signal pathway in autoimmune arthritis. EBioMedicine 2019;43:620-31. [Crossref] [PubMed]
- Kong N, Lan Q, Su W, et al. Induced T regulatory cells suppress osteoclastogenesis and bone erosion in collagen-induced arthritis better than natural T regulatory cells. Ann Rheum Dis 2012;71:1567-72. [Crossref] [PubMed]
- Bézie S, Picarda E, Ossart J, et al. IL-34 is a Treg-specific cytokine and mediates transplant tolerance. J Clin Invest 2015;125:3952-64. [Crossref] [PubMed]
- Zheng SG, Wang JH, Gray JD, et al. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol 2004;172:5213-21. [Crossref] [PubMed]
- Zheng SG, Wang J, Wang P, et al. IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol 2007;178:2018-27. [Crossref] [PubMed]
- Zheng SG, Gray JD, Ohtsuka K, et al. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25- precursors. J Immunol 2002;169:4183-9. [Crossref] [PubMed]
- Liu Y, Lan Q, Lu L, et al. Phenotypic and functional characteristic of a newly identified CD8+ Foxp3- CD103+ regulatory T cells. J Mol Cell Biol 2014;6:81-92. [Crossref] [PubMed]
- Gao Y, Tang J, Chen W, et al. Inflammation negatively regulates FOXP3 and regulatory T-cell function via DBC1. Proc Natl Acad Sci U S A 2015;112:E3246-54. [Crossref] [PubMed]
- Li B, Zheng SG. How regulatory T cells sense and adapt to inflammation. Cell Mol Immunol 2015;12:519-20. [Crossref] [PubMed]
- Xu A, Liu Y, Chen W, et al. TGF-beta-Induced Regulatory T Cells Directly Suppress B Cell Responses through a Noncytotoxic Mechanism. J Immunol 2016;196:3631-41. [Crossref] [PubMed]
- Zhou X, Wang J, Shi W, et al. Isolation of purified and live Foxp3+ regulatory T cells using FACS sorting on scatter plot. J Mol Cell Biol 2010;2:164-9. [Crossref] [PubMed]
- Su W, Fan H, Chen M, et al. Induced CD4+ forkhead box protein-positive T cells inhibit mast cell function and established contact hypersensitivity through TGF-beta1. J Allergy Clin Immunol 2012;130:444-52.e7. [Crossref] [PubMed]
- Nakamichi Y, Udagawa N, Takahashi N. IL-34 and CSF-1: similarities and differences. J Bone Miner Metab 2013;31:486-95. [Crossref] [PubMed]
- Zhu SL, Huang JL, Peng WX, et al. Inhibition of smoothened decreases proliferation of synoviocytes in rheumatoid arthritis. Cell Mol Immunol 2017;14:214-22. [Crossref] [PubMed]
- Liu Y, Pan YF, Xue YQ, et al. uPAR promotes tumor-like biologic behaviors of fibroblast-like synoviocytes through PI3K/Akt signaling pathway in patients with rheumatoid arthritis. Cell Mol Immunol 2018;15:171-81. [Crossref] [PubMed]
- Mo BY, Guo XH, Yang MR, et al. Long Non-Coding RNA GAPLINC Promotes Tumor-Like Biologic Behaviors of Fibroblast-Like Synoviocytes as MicroRNA Sponging in Rheumatoid Arthritis Patients. Front Immunol 2018;9:702. [Crossref] [PubMed]
- Chen W, Wang J, Xu Z, et al. Apremilast Ameliorates Experimental Arthritis via Suppression of Th1 and Th17 Cells and Enhancement of CD4(+)Foxp3(+) Regulatory T Cells Differentiation. Front Immunol 2018;9:1662. [Crossref] [PubMed]
- Zou Y, Xu S, Xiao Y, et al. Long noncoding RNA LERFS negatively regulates rheumatoid synovial aggression and proliferation. J Clin Invest 2018;128:4510-24. [Crossref] [PubMed]
- Yu G, Bing Y, Zhu S, et al. Activation of the interleukin-34 inflammatory pathway in response to influenza A virus infection. Am J Med Sci 2015;349:145-50. [Crossref] [PubMed]
- Wang YQ, Cao WJ, Gao YF, et al. Serum interleukin-34 level can be an indicator of liver fibrosis in patients with chronic hepatitis B virus infection. World J Gastroenterol 2018;24:1312-20. [Crossref] [PubMed]
- Cheng ST, Tang H, Ren JH, et al. Interleukin-34 inhibits hepatitis B virus replication in vitro and in vivo. PLoS One 2017;12:e0179605 [Crossref] [PubMed]
- Preisser L, Miot C, Le Guillou-Guillemette H, et al. IL-34 and Macrophage Colony-Stimulating Factor Are Overexpressed in Hepatitis C Virus Fibrosis and Induce Profibrotic Macrophages That Promote Collagen Synthesis by Hepatic Stellate Cells. Hepatology 2014;60:1879-90. [Crossref] [PubMed]
- Mathews S, Woods AB, Katano I, et al. Human Interleukin-34 facilitates microglia-like cell differentiation and persistent HIV-1 infection in humanized mice. Mol Neurodegener 2019;14:12. [Crossref] [PubMed]
- Ye C, Brand D, Zheng SG. Targeting IL-2: an unexpected effect in treating immunological diseases. Signal Transduct Target Ther 2018;3:2. [Crossref] [PubMed]
- Pan HF, Leng RX, Feng CC, et al. Expression profiles of Th17 pathway related genes in human systemic lupus erythematosus. Mol Biol Rep 2013;40:391-9. [Crossref] [PubMed]
- Li N, Wang JC, Liang TH, et al. Pathologic finding of increased expression of interleukin-17 in the synovial tissue of rheumatoid arthritis patients. Int J Clin Exp Pathol 2013;6:1375-9. [PubMed]
- Huang Z, Fu B, Zheng SG, et al. Involvement of CD226+ NK cells in immunopathogenesis of systemic lupus erythematosus. J Immunol 2011;186:3421-31. [Crossref] [PubMed]
- Rosenblum MD, Remedios KA, Abbas AK. Mechanisms of human autoimmunity. J Clin Invest 2015;125:2228-33. [Crossref] [PubMed]
- Xie HH, Shen H, Zhang L, et al. Elevated Serum Interleukin-34 Level in Patients with Systemic Lupus Erythematosus Is Associated with Disease Activity. Sci Rep 2018;8:3462. [Crossref] [PubMed]
- Xi R, Fan Q, Yan X, et al. Increased Serum Interleukin-34 Levels Are Related to the Presence and Severity of Cardiac Dysfunction in Patients With Ischemic Cardiomyopathy. Front Physiol 2018;9:904. [Crossref] [PubMed]
- Kuzumi A, Yoshizaki A, Toyama S, et al. Serum interleukin-34 levels in patients with systemic sclerosis: Clinical association with interstitial lung disease. J Dermatol 2018;45:1216-20. [Crossref] [PubMed]
- Zorena K, Jachimowicz-Duda O, Waz P. The cut-off value for interleukin 34 as an additional potential inflammatory biomarker for the prediction of the risk of diabetic complications. Biomarkers 2016;21:276-82. [Crossref] [PubMed]
- Shoji H, Yoshio S, Mano Y, et al. Interleukin-34 as a fibroblast-derived marker of liver fibrosis in patients with non-alcoholic fatty liver disease. Sci Rep 2016;6:28814. [Crossref] [PubMed]
- Tian Y, Shen H, Xia L, et al. Elevated serum and synovial fluid levels of interleukin-34 in rheumatoid arthritis: possible association with disease progression via interleukin-17 production. J Interferon Cytokine Res 2013;33:398-401. [Crossref] [PubMed]
- Chang SH, Choi BY, Choi J, et al. Baseline serum interleukin-34 levels independently predict radiographic progression in patients with rheumatoid arthritis. Rheumatol Int 2015;35:71-9. [Crossref] [PubMed]
- Chemel M, Le Goff B, Brion R, et al. Interleukin 34 expression is associated with synovitis severity in rheumatoid arthritis patients. Ann Rheum Dis 2012;71:150-4. [Crossref] [PubMed]
- Moon SJ, Hong YS, Ju JH, et al. Increased levels of interleukin 34 in serum and synovial fluid are associated with rheumatoid factor and anticyclic citrullinated peptide antibody titers in patients with rheumatoid arthritis. J Rheumatol 2013;40:1842-9. [Crossref] [PubMed]
- Kawabe M, Ohyama H, Kato-Kogoe N, et al. Expression of interleukin-34 and colony stimulating factor-1 in the stimulated periodontal ligament cells with tumor necrosis factor-alpha. Med Mol Morphol 2015;48:169-76. [Crossref] [PubMed]
- Hussein YAA, Sadeq Y. Impact of IL34, IFNa and IFN-lambda 1 on disease activity of SLE patients in EGYPT. Ann Rheum Dis 2019;78:405.
- Zhang X, Ouyang X, Xu Z, et al. CD8+CD103+ iTregs Inhibit Chronic Graft-versus-Host Disease with Lupus Nephritis by the Increased Expression of CD39. Mol Ther 2019;27:1963-73. [Crossref] [PubMed]
- Xiao ZX, Zheng X, Hu L, et al. Immunosuppressive Effect of B7-H4 Pathway in a Murine Systemic Lupus Erythematosus Model. Front Immunol 2017;8:1765. [Crossref] [PubMed]
- Ma J, Yu J, Tao X, et al. The imbalance between regulatory and IL-17-secreting CD4+ T cells in lupus patients. Clin Rheumatol 2010;29:1251-8. [Crossref] [PubMed]
- Jacob N, Yang H, Pricop L, et al. Accelerated pathological and clinical nephritis in systemic lupus erythematosus-prone New Zealand Mixed 2328 mice doubly deficient in TNF receptor 1 and TNF receptor 2 via a Th17-associated pathway. J Immunol 2009;182:2532-41. [Crossref] [PubMed]
- Zhao P, Wang P, Dong S, et al. Depletion of PD-1-positive cells ameliorates autoimmune disease. Nat Biomed Eng 2019;3:292-305. [Crossref] [PubMed]
- Shaban E, Bayliss G, Malhotra DK, et al. Targeting Regulatory T Cells for Transplant Tolerance: New Insights and Future Perspectives. Kidney Dis (Basel) 2018;4:205-13. [Crossref] [PubMed]
- Rosen SJ, Harris PE, Hardy MA. State of the Art: Role of the Dendritic Cell in Induction of Allograft Tolerance. Transplantation 2018;102:1603-13. [Crossref] [PubMed]
- Zheng SG, Meng L, Wang JH, et al. Transfer of regulatory T cells generated ex vivo modifies graft rejection through induction of tolerogenic CD4+CD25+ cells in the recipient. Int Immunol 2006;18:279-89. [Crossref] [PubMed]
- Nakamura T, Ushigome H. Myeloid-Derived Suppressor Cells as a Regulator of Immunity in Organ Transplantation. Int J Mol Sci 2018; [Crossref] [PubMed]
- Camirand G, Riella LV. Treg-Centric View of Immunosuppressive Drugs in Transplantation: A Balancing Act. Am J Transplant 2017;17:601-10. [Crossref] [PubMed]
- Lindau R, Mehta RB, Lash GE, et al. Interleukin-34 is present at the fetal-maternal interface and induces immunoregulatory macrophages of a decidual phenotype in vitro. Hum Reprod 2018;33:588-99. [Crossref] [PubMed]
- Foucher ED, Blanchard S, Preisser L, et al. IL-34 induces the differentiation of human monocytes into immunosuppressive macrophages. antagonistic effects of GM-CSF and IFNgamma. PLoS One 2013;8:e56045 [Crossref] [PubMed]
- Liao T, Xue Y, Zhao D, et al. In Vivo Attenuation of Antibody-Mediated Acute Renal Allograft Rejection by Ex Vivo TGF-beta-Induced CD4(+)Foxp3(+) Regulatory T Cells. Front Immunol 2017;8:1334. [Crossref] [PubMed]
- Zhang X, Huang F, Li W, et al. Human Gingiva-Derived Mesenchymal Stem Cells Modulate Monocytes/Macrophages and Alleviate Atherosclerosis. Front Immunol 2018;9:878. [Crossref] [PubMed]
- Kadam PD, Chuan HH. Erratum to: Rectocutaneous fistula with transmigration of the suture: a rare delayed complication of vault fixation with the sacrospinous ligament. Int Urogynecol J 2016;27:505. [Crossref] [PubMed]
- Cai W, Wang J, Hu M, et al. All trans-retinoic acid protects against acute ischemic stroke by modulating neutrophil functions through STAT1 signaling. J Neuroinflammation 2019;16:175. [Crossref] [PubMed]
- Cai W, Liu S, Hu M, et al. Post-stroke DHA Treatment Protects Against Acute Ischemic Brain Injury by Skewing Macrophage Polarity Toward the M2 Phenotype. Transl Stroke Res 2018;9:669-80. [Crossref] [PubMed]
- Xie C, Zhu F, Wang J, et al. Off-Target Deletion of Conditional Dbc1 Allele in the Foxp3(YFP-Cre) Mouse Line under Specific Setting. Cells 2019; [Crossref] [PubMed]
- Su W, Chen X, Zhu W, et al. The cAMP-Adenosine Feedback Loop Maintains the Suppressive Function of Regulatory T Cells. J Immunol 2019;203:1436-46. [Crossref] [PubMed]
Cite this article as: Hu X, Huang F, Deng Y, Wang J, Wang J. The multifactorial roles of IL-34 in immune responses. Ann Blood 2020;5:4.