
Platelet physiology and immunology: pathogenesis and treatment of classical and non-classical fetal and neonatal alloimmune thrombocytopenia
Introduction
Platelets, thrombocytes, and thrombocytopenia
Platelets are anucleate blood cells derived from megakaryocyte cells in the bone marrow of mammals (1,2). Interestingly, recent studies have demonstrated that platelets could also be produced in lungs (3), however the portion of these lung-derived platelets in blood circulation and whether they possess different and/or additional functions are still under debate. Platelets have been known for more than a century for their critical functions in hemostasis and thrombosis (4,5). Even so, continuing research in the last few decades, particularly last few years, reveals platelets to be versatile and significantly contribute to many other physiological and pathological processes such as atherosclerosis (6,7), angiogenesis (8,9), tumor metastasis (10), lymphatic vessel development (11,12) and liver regeneration (13,14). Importantly, platelets are considered as an important part of the immune system and actively engage in both innate and adaptive immune responses via their surface adhesion molecules and intracellular components (15-17). For instance, platelets demonstrate “innate immune cell-like” roles by possessing rudimentary phagocytic and antimicrobial activity (15,16), and releasing pro-inflammatory cytokines such as interleukin-1 (IL-1) during infections (15,16,18). Platelets may also have anti-inflammatory and immune regulatory functions through the release of immunosuppressive cytokines such as transforming growth factor beta (TGF-β) and IL-10, although more evidence is required to establish their dual roles in immune responses (15).
In lower (non-mammalian) vertebrates, thrombocytes are nucleated blood cells which play similar hemostatic and immune protective roles, suggesting that platelet functions in mammals are important and evolutionarily conserved (1,15,16,19).
Thrombocytopenia, defined as a low number of thrombocytes or platelets in the body, is a hematologic disorder which can be inherited or acquired (4). When not properly treated, this may result in severe bleeding diathesis and become life-threatening. The typical acquired thrombocytopenia result from the immune system targeting platelets for destruction, which can occur in either autoimmune or alloimmune disorders such as immune thrombocytopenia (ITP) and fetal and neonatal alloimmune thrombocytopenia (FNAIT), respectively (20-22).
FNAIT, the most common cause of severe thrombocytopenia in both fetuses and neonates (22), will be the main focus of this review, particularly elaborating on non-classical FNAIT such as early miscarriage and intrauterine growth restriction (IUGR), as well as intracranial hemorrhage (ICH). In order to better understand the complex pathogenesis of FNAIT, especially for the non-classical FNAIT, and develop better treatments, it is important to take into account that platelets are versatile and play the roles of both passive targets and active immune regulators beyond their well-recognized function in arrest of bleeding. Following, we will discuss the roles of platelets in hemostasis as well as mutual interactions between platelets and the immune system.
Platelets play central roles in hemostasis: old topic with new discoveries
Platelets are small cells or “fragments” of megakaryocytes. After being released into the blood, the physical force of larger red blood cells “pushes” platelets aside, maintaining a majority of them in close proximity to the vessel wall (1,2,5). At the site of vascular injury, subendothelial matrix proteins such as collagen are exposed to the blood flow, which anchors von Willebrand factor (VWF), and initiates platelet glycoprotein (GP) GPIbα-VWF interaction. This advances platelet tethering/adhesion, particularly at high shear conditions such as the area of arterial stenosis (23-25). GPIbα-VWF transient interactions mediate platelet rolling and translocation onto the injured vessel wall, slowing them down, and provide the opportunity for subsequent GPVI-collagen interactions, an important step for further activation of platelets (26,27). In addition to GPIbα-VWF interaction, some soluble factors released from platelets or generated from blood coagulation, such as ADP and thrombin, can also deliver signals for platelet activation (24,28-30). These activated platelets then express active conformations of integrins such as αIIbβ3 integrin (GPIIbIIIa) (31-33). Through interactions with their ligands on the vessel wall such as VWF, fibrinogen/fibrin, collagen, fibronectin, laminins, and thrombospondin-1, these integrins (αIIbβ3, likely also αVβ3 and α2β1, α5β1 α6β1) can mediate firm platelet adhesion onto the injured vessel wall (31,34-36). Under low shear conditions such as in veins, these integrin-ligand interactions may be sufficient to initiate platelet adhesion (23,31).
Following the first layer of platelet adhesion, the aggregation between adjacent platelets can occur through the binding of plasma fibrinogen to activated αIIbβ3 integrins (37-39). Interestingly, although the theory that fibrinogen is required for platelet aggregation has been established for more than 50 years, platelet aggregation persists in mice lacking fibrinogen, fibrinogen and VWF, and even triple deficient fibrinogen/VWF/plasma fibronectin (40,41), but does not occur in β3 deficient mice (42). These results can be further demonstrated in vitro in aggregometry using non-anticoagulated (but not anticoagulated) blood (41-43). Thus, anti-coagulant agents have masked the fibrinogen-independent platelet aggregation for the last half-century (39,42). These findings shift the paradigm in the field and provide insight into the mechanisms which support the survival of some patients with afibrinogenemia (44,45). Since αIIbβ3 is required and both plasma and platelet proteins contribute to this platelet aggregation (42), it is worthwhile to further identify these αIIbβ3 ligands and characterize their roles in hemostasis (45-50).
Platelet accumulation (adhesion and aggregation) at the site of vascular injury has been considered as the first wave of hemostasis. The second wave is mediated by blood coagulation, which generates thrombin via extrinsic and/or intrinsic pathways (20,23,29,30). Thrombin converts the soluble fibrinogen to insoluble fibrin, leading to blood coagulation and hemostasis (51,52). Notably, there are many interactions between the first wave and the second wave of hemostasis. For example, thrombin generated from blood coagulation (the second wave) is the most potent platelet agonist triggering platelet activation, enhancing platelet adhesion/aggregation (the first wave). On the other hand, activated platelets may generate phosphatidylserine (PS) on their surfaces, harboring the coagulation factors that markedly enhance cell-based thrombin generation (23,53,54). Understanding this process is useful for us to explore the possible mechanisms of fibrin and thrombus formation in placenta induced by maternal anti-fetal platelet antibodies in FNAIT (55).
Interestingly, we recently observed a “protein wave of hemostasis”, which occurs even prior to platelet accumulation (the first wave) (23,43). We found plasma fibronectin plays previously unreported hemostatic roles. Although it by itself does not support, but rather, inhibits platelet aggregation (41,43), plasma fibronectin can covalently link to fibrin and subsequently support platelet aggregation (43,45,56). Most importantly, plasma fibronectin can quickly deposit onto the injured vessel wall, likely via interaction with collagen or other proteins, and form a hemostatic matrix (the protein wave) (43). Since there is a 3-5 fold increase of plasma fibronectin content in platelets from fibrinogen deficient/γ chain mutant mice and afibrinogenemic patients (40,44,45,57), these platelets may release fibronectin from their α granules and deliver them into the site of injury to compensate for the bleeding disorder in afibrinogenemic conditions, although more evidence is required to establish this compensatory pathway. Thus, platelets contribute to both first and the second waves as well as the newly termed “protein wave” of hemostasis (20,23,43).
It is notable that the levels and function of plasma fibrinogen and other coagulation factors as well as platelets may have considerable differences in fetuses compared to adults (58,59). Understanding these differences should be very useful for apprehending the bleeding disorders and other symptoms of FNAIT.
Thrombocytopenia: autoimmune and alloimmune bleeding disorders
Multiple genetic and environmental factors, including those that are malignancy-associated and immune-mediated, may contribute to the impairment of platelet production and clearance, leading to thrombocytopenia (4,15,60,61). Inherited thrombocytopenias include genetic defects leading to abnormal platelet size and function (62,63), and/or impaired platelet production (i.e., micro-, normo-, macrothrombocytopenia) (63). Malignancy-associated thrombocytopenias arise due to underlying conditions (e.g., chronic lymphocytic leukemia and lymphomas, breast, and ovarian cancers, etc.) (64,65). Immune-mediated thrombocytopenias, which can be further classified as autoimmune or alloimmune thrombocytopenia, result from an abnormal immune response against either one’s own platelets or other sources of platelet alloantigens (21,66,67).
Autoimmune thrombocytopenia includes primary thrombocytopenia (ITP) and secondary thrombocytopenia, which may be induced after infection (e.g., HIV, HCV) or drugs (e.g., heparin) (60). While most thrombocytopenic patients are asymptomatic, some can experience a wide range of bleeding conditions such as petechiae, mucosal bleeding, epistaxes, and/or menorrhagia (4). Most concerning is the risk of ICH which is estimated to occur in 1.5–1.8% of adult ITP patients and cause fatality in 25% of these cases (4,68). While it was previously known that autoantibodies against platelet antigens opsonized the platelet to be engulfed by Fc-receptor (FcR) expressing phagocytes (e.g., macrophages) and cleared in the spleen (69,70), recent research revealed an antibody Fc-independent pathway (71-73), as well as the involvement of CD8+ cytotoxic T lymphocytes (CTL) in the disease process (74-76). Notably, CD8+ T regulatory cells have also been identified and may play some unique roles (77), synergizing with CD4+ T regulatory cells during immune-mediated thrombocytopenia (77,78).
Whether an Fc-dependent or independent immune response in ITP is dependent on antigen specificities is a hot topic in the field (71-73,79-82). GPIbα complex and GPIIbIIIa (αIIbβ3 integrin), the two key platelet receptors for VWF and fibrinogen, are the major autoantigens in ITP. These two receptors belong to distinctive protein families, which have their own unique signal pathways affecting platelet activities and likely eliciting different immune responses (83,84). Earlier studies found that anti-GPIbα monoclonal antibodies can induce thrombocytopenia without their Fc portions or Fc receptor (FcR) (i.e., FcγR) in mice (71,72). Subsequent studies demonstrated that anti-GPIbα-antibody-mediated thrombocytopenia is not sensitive to intravenous IgG (IVIG) therapies in both mice and human ITP patients (72,85,86), which is consistent with the prevailing mechanism of FcR blockage by IVIG (87-89). Interestingly, it is also not sensitive to steroid therapies (90). Thus, anti-GPIbα-mediated ITP seems to have 2–3 times higher likelihood of being refractory to the first line therapies (i.e., steroid and IVIG) compared with anti-αIIbβ3-antibody-mediated ITP (86,90). Recent studies demonstrated that some anti-GPIbα antibodies can induce conformational changes in GPIbα mechanosensory domain, which delivers signals for platelet activation (81), causes platelet desialylation (79,80,91) and apoptosis (55,79,92), leading to platelet clearance likely by Kupffer cells in the liver (80,93). Notably, some anti-αIIbβ3 antibodies can also induce platelet apoptosis (89) and desialylation, particularly in humans, since the FcR on human platelets can crosslink with these autoantibody Fc portions and provide additional signals for platelets (80). Whether this type of anti-αIIbβ3-mediated ITP is also refractory for the first line therapies and/or splenectomy remains to be further investigated. The available data suggests that platelet desialylation is inversely correlated with efficacy of the first-line therapies (94) and sialidase inhibitors may be useful to ameliorate thrombocytopenia (95-98).
There is no doubt that there are significant similarities for platelet opsonization and clearance between autoimmune- and alloimmune thrombocytopenias (e.g., ITP and FNAIT). Platelets, including platelet released cytokines, likely affect the immune responses and the disease processes in both ITP and FNAIT (15,99). However, immune response/reactions in pregnant women to alloantigens on fetal platelets, particularly in the context of maternal immune tolerance, may have considerable differences, and little information is available today for the reticuloendothelial system (RES) in fetuses. In addition, it has not been addressed whether different autoantibodies (Fc-dependent versus Fc-independent) in pregnant women with ITP may differently affect their fetuses and fetal platelet clearance. These interesting questions should be further studied in the near future.
FNAIT
In alloimmune thrombocytopenia, antibody-mediated platelet destruction may occur following transfusions of platelets derived from genetically different donors (termed post-transfusion purpura; PTP), or during pregnancy against paternally-derived platelet antigens (FNAIT) (66). Unlike hemolytic disease of the fetus and newborn (HDFN), a condition where antigens on fetal red blood cells are targeted by maternal alloantibodies, FNAIT may develop in the first pregnancy in 50% of cases, and close to 100% of subsequent pregnancies that have siblings who are similarly platelet-antigen positive (100-102). It is likely that the antigen targeted, the accessibility of location, and length of exposure are all critical factors that influence the pathogenesis of disease. In comparison to ITP patients, fetuses and neonates can face 10-100 fold increased risk of severe bleeding conditions (103). Life-threatening complications include ICH, IUGR, and neurological sequelae (66,100,104). Murine models developed by our laboratory has revealed new insights into the pathogenesis of FNAIT (55,88,102,105-107), however the mechanisms leading to miscarriage are still largely unknown (108-110). Here, we will discuss the “classical” FNAIT that manifest with thrombocytopenia and fetal/neonatal bleeding, and the “non-classical” FNAIT that do not present with typical bleeding symptoms and have not been well described or recognized in the field.
Alloantigens in FNAIT
There are 37 human platelet antigens (HPAs) that have been defined by Platelet Nomenclature Committee to be targeted in FNAIT (Figure 1), that are spread across six platelet surface proteins: αIIb (GPIIb), β3 (GPIIIa), and α2 (GPIa), GPIbα, GPIbβ, and cluster of differentiation CD109 (101,111-113). When referring to HPAs, “a” following the number indicates high frequency forms, and “b” for low frequency forms (114). Amongst the HPAs targeted in FNAIT, more than 80% of FNAIT cases in Caucasians are targeting the extracellular domain of β3 subunit (115,116), and less than 20% of the cases are targeting other platelet surface proteins. Interestingly, although integrin subunit αIIb, which pairs exclusively with β3, has a similar number of polymorphisms as β3 (117), it is not understood why there is a much lower reported incidence of anti-αIIb mediated FNAIT. Moreover, there are <1% of reports on anti-GPIbα (e.g., HPA-2) mediated FNAIT, in contrast to 20–40% prevalence of anti-GPIbα complex antibodies reported for ITP patients (102,118). Ongoing studies from animal models suggest that the rarity of anti-αIIb and anti-GPIbα-mediated FNAIT may be attributed to life-threatening phenotypes, resulting in underreported miscarriages (55,119-121).
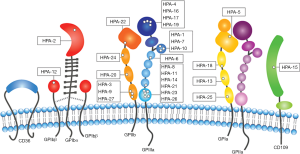
Certain single nucleotide polymorphisms (SNPs) on the same integrin may elicit different severities of immune responses. While HPA-1a-mediated alloimmune response (Leu to Pro substitution at residue 33 of β3) is attributed to >75% of the FNAIT cases in people with mixed European descent (66,101,122), this polymorphism is relatively rare in both African and Asian populations (102). In comparison, HPA-4b mediated FNAIT (Gln to Arg substitution at residue 143 of β3) is more prevalent in Japan (123). Likewise, HPA-2a (Thr to Met substitution on reside 145 on GPIbα) and HPA-3a (Ile to Ser substitution on residue 843 on αIIb) are not frequently reported as targets of FNAIT in Caucasian populations (113,118,119). Structural analysis into the SNPs which are responsible for HPAs reveals that many of them are exposed on the surface of the protein, far away from the ligand binding site (124). However, some anti-HPAs associated with impairment of ligand binding sites are found (101,102). It is speculated that single amino acid substitutions may lead to changes in charges and electrostatic potential of the receptor, and contribute to changes in its antigenicity (124). Although allele frequencies within certain ethnic groups may allow us to predict whether an incompatibility might arise, incompatibility does not necessarily correspond with the incidence of disease.
While not formally designated as an HPA in FNAIT, anti-CD36 mediated FNAIT is common in Chinese, Japanese, and other Asian populations, but have not been reported in Caucasian populations (125,126). One explanation could be a lack of CD36 on the surface of platelets and/or monocytes which is prevalent in 2–5% of Asian populations compared to less than 0.3% of Caucasians (126). While CD36 deficiency has not been definitely linked to a disease, the roles of CD36 in diseases have been reported (127-130). In the case of FNAIT, CD36 deficiency may lead to the risk to develop isoantibodies against the entire CD36 protein (125,131,132). Future studies assessing the frequency of anti-CD36 in different ethnic groups requires improved international collaboration, and aids in better understanding the genetic and environmental influences of anti-CD36 mediated FNAIT and other anti-CD36 mediated alloimmune disorders (101,126,133).
Alloantigen presentation and antibody generation
There may be multiple opportunities before, during, and after pregnancy for an alloimmune response to occur. For instance, some women are pre-exposed to HPAs prior to pregnancy (i.e., through semen, blood transfusions) which could lead to the development of alloantibodies and predispose the fetus to develop FNAIT (66,88). Whether alloantibodies against platelets cross-react with semen, or vice versa, and lead to difficulties in conception and FNAIT is currently unknown. In the event of pregnancy, it is unclear how fetal platelet antigens trigger the maternal immune system, considering that the placenta is an immunoprivileged site and the mother is in a “physiological immune tolerance” condition. However, fetal microparticles have been detected in maternal serum as early as 7 weeks (134-136), thereby becoming a potential source of foreign antigens for the maternal immune system to generate antibodies against. Notably, the trophoblast cells of the placenta, which express fetal polymorphic platelet surface proteins, may be another accessible source of alloantigens (106,137-139).
Fetal alloantigens can be processed by maternal antigen presenting cells (APCs) such as dendritic cells and macrophages, and subsequently presented to naïve CD4 T helper (Th) cells. These T cells become activated, proliferate, and release cytokines which can further induce B cells to differentiate and produce anti-fetal platelet antigen antibodies (66). The ability of an antigen to be presented to a naïve T cell depends on the affinity of the major histocompatibility complex (MHC) allele of the APC to the antigen. For instance, women carrying the MHC allele DRB3*01:01 allele are 25 times more likely to develop alloantibodies against HPA-1a compared to women who lack this allele. In one study, 90% of immunized HPA-1a negative women that were DRB3*01:01 positive had significantly increased antibody titers compared to DRB3*01:01 negative women (140,141). In a retrospective study, male neonates who were found with anti-HPA-1a antibodies experienced on average significantly lower than average birth weights. A similar, although non-significant negative correlation existed for FNAIT-affected females (142). While these data support the notion that male sex is an independent risk factor for adverse pregnancy outcomes (143), more research is required to better understand how offspring sex is involved in FNAIT (142). It is important to note, however, that given its frequency in the Caucasian population, HPA-1a-mediated FNAIT has been studied more in depth than any other HPAs. It is currently unknown whether other HPA antigens are processed and presented less efficiently to antigen-specific T helper cells, and whether this is linked to differences in certain anti-HPA antibody titers (66,101).
Antibody cross-placental transport via neonatal FcRn
Maternal alloantibodies against paternally-derived platelet antigens can be detected in fetal circulation at approximately 13 weeks gestation in humans and embryonic day (E) 10.5 in mice (143-145). Using β3 integrin and neonatal FcRn deficient murine models, we demonstrated that FcRn expressed by the syncytiotrophoblast cells of the outermost layer chorionic villi of the placenta, not maternal FcRn, is required for the transfer of maternal IgG from mother to fetus (105). This leads to several interesting questions which remain unanswered such as: (I) where and how IgG-FcRn interaction occurs in the placenta; (II) whether human FcRn polymorphisms may result in different affinities of maternal IgG transport across the placenta and (III) if this mitigates the likelihood or severity of FNAIT development during pregnancy. Our preliminary results also show that FNAIT symptoms can be ameliorated through the use of fetal FcRn blockade, thus arising the potential to develop into a novel FNAIT therapeutic intervention (105).
Antibody-mediated fetal thrombocytopenia
Research efforts in the past few decades have elucidated several distinct antibody-mediated mechanisms that contribute to the pathogenesis of thrombocytopenias. Generally, the mechanisms of platelet destruction and clearance in FNAIT is assumed to be similar as ITP: the Fc portion of the alloantibody that is bound to fetal platelet antigens interacts with the FcRs (e.g., FcγRIIa, FcγIIIa) expressed by macrophages, initiating phagocytosis and clearance of platelets in the RES of the spleen. Recently, it was demonstrated in mice that fetal macrophage populations are heterogeneous, for instance, CD11bHI macrophage from fetal liver released pro-inflammatory cytokines in response to antigens such lipopolysaccharide (LPS) while low CD11bHI macrophages derived from yolk sac were non-responsive (146). These findings provide new insight into fetal immunity especially within an immunosuppressive environment as the placenta. Indeed, how these pro-inflammatory macrophages respond to maternal antibodies, especially in the context of FNAIT, is unknown. In addition, as previously mentioned, in ITP murine models, we have shown an Fc-independent mechanism where anti-GPIbα antibodies cause platelet activation and desialylation, subsequently leading to clearance in the liver (80,147). However it is not well understood whether fetuses also clear platelets via the same pathways.
Other immune-cell involvement of FNAIT pathogenesis
While other immune cell involvement has not been extensively studied on the fetal-maternal interface in the context of FNAIT, it is clear that maternal immune competent (e.g., APCs, T and B) cells are involved in the immune response against fetal platelet antigens. Research on ITP found that CD4+ helper T cells secrete immune regulating cytokines (e.g., IL-2) and can proliferate in vitro when stimulated with autologous platelets (75,76) while CD8+ CTLs may mediate platelet lysis (148). Whether these cells also contribute to damage antigen positive trophoblasts in FNAIT and impairment of placenta development remains to be further studied. In addition, how maternal CD4+ and CD8+ T regulatory cells impact pro-inflammatory immune responses during pregnancy and affect placenta development requires further investigation (66). The immune reactions in the fetal RES system have not been adequately explored; the roles of fetal spleen/liver macrophages, as well as other immune competent cells in FNAIT are of interest in future studies.
Non-classical FNAIT: mechanisms of miscarriage
The “classical” FNAIT is characterized as fetal and neonatal thrombocytopenia along with bleeding disorders. However, since platelets are versatile and platelet alloantigens may expressed on other cells, anti-platelet responses may therefore lead to different pathogenesis from the classical FNAIT. These “non-classical” FNAIT symptoms have been observed in our animal models, which do not present with typical bleeding and have not been well described or recognized in the field. We will introduce some of them here.
Anti-GPIbα-mediated thrombosis in the placenta
GPIbα is one of the major antibody targets in ITP, and approximately 20–40% serum positive patients have anti-GPIbα antibodies. Interestingly, only a few cases of FNAIT have been linked with anti-GPIbα antibodies. The rarity of anti-GPIbα-mediated FNAIT cannot be explained by the frequency of HPA-2 polymorphism on GPIbα (101,149). Utilizing both immunized GPIbα and β3 deficient mice as active murine models of FNAIT, we discovered that miscarriage occurred in >83% of pregnant mice with anti-GPIbα antibodies, which is far more frequent than those pregnant mice with anti-β3 antibodies (55). Interestingly, these anti-GPIbα-mediated miscarriages were not observed with bleeding symptoms. Instead, anti-GPIbα caused a previously unidentified, non-classical FNAIT where anti-GPIbα antibodies activated platelets (i.e., platelet calcium mobilization, enhanced PS expression, enhanced cell-based thrombin generation), leading to fibrin deposition in the placenta, blockage of fetal blood supply and apoptosis/necrosis in the placenta (55). These data are consistent with recent observations in ITP studies (79-81). Collectively, our results demonstrated that differences in FNAIT pathophysiology may depend on the platelet antigen targeted.
Whether increased miscarriages caused by anti-GPIbα antibodies accounts for the rarity of reported human anti-GPIbα-mediated FNAIT remains unknown (66). Notably, similar to anti-β3-mediated FNAIT, in our murine studies, IVIG therapy or anti-FcRn antibodies were able to efficiently prevent anti-GPIbα-mediated FNAIT by blocking FcRn and preventing pathogenic maternal antibodies to cross the placenta (55). Screening GPIbα polymorphisms for women who experience frequent miscarriages and detecting anti-GPIbα antibodies may be useful strategies to identify and assess the risks of miscarriage (150-152). Recall this is unlike anti-GPIbα-mediated ITP murine models where IVIG was not as effective in ameliorating thrombocytopenia due to an Fc-independent pathway (78,80). However, it is still unknown whether downstream platelet clearance by anti-GPIbα in FNAIT is also mediated by an Fc-independent pathway in the fetal RES system. The efficacy of IVIG has not yet been translated to human patients with anti-GPIb-mediated FNAIT due to its rarity.
Impairment of angiogenesis by targeting β3 integrin
One of the most serious complications of FNAIT is ICH (66). On average, 10–20% of neonates born with FNAIT experience ICH, and approximately 5% of these ICH cases are fatal (66,102). In fetuses, ICH can be detected before 20 weeks of age, and about 10% of these fetuses may develop permanent neurological impairment (66,101,153).
While it is generally thought that fetal thrombocytopenia caused by maternal alloantibodies is the main cause of ICH seen in FNAIT, emerging research suggests there are other factors mediating ICH. Genetically engineered mice lacking circulating platelets (deficient for the hematopoietic subunit of the heterodimeric erythroid transcription factor NF-E2) do not experience ICH when delivered by caesarian section nor significant fetal death (154). Additionally, a lack in the ability to form fibrin clots in fetuses and neonates was not the major cause of observed hemorrhages, as shown by fibrinogen deficient mice (41,42,155). Even more strikingly, the fetuses with double deficient of NF-E2 and fibrinogen exhibited normal embryonic development, were morphologically indistinguishable from their wild-type controls, and experienced no obvious bleeding (58).Taken together, current data stimulate another “non-classical” approach describing mechanisms of bleeding in FNAIT beyond thrombocytopenia and/or blood coagulation.
These findings prompted further research into the mechanisms behind ICH. Integrin β3 (GPIIIa) subunit, targeted in many FNAIT cases, can be expressed with αIIb in the GPIIbIIIa complex, or with αV as a part of vitronectin receptors widely prevalent on endothelial cells and several other cell types (156,157). Our group found anti-β3 antibodies may also target endothelial cells and impair angiogenesis in a murine model of FNAIT (158), contributing to haemorrhage during embryogenesis and fetal growth (107,159). Impaired development of angiogenic signaling and other abnormalities in angiogenesis are linked to severe hemorrhage in the brain, and has been demonstrated in both zebra fish and rabbit models, respectively (160,161). Indeed, the brain, one of the most active angiogenic organs during the fetal growth, may be significantly impacted as a consequence of anti-β3 antibodies, leading to the development of ICH (162-164). Our laboratory demonstrated that fetuses and neonates of immunized β3-/- murine models experienced ICH and impaired retinal angiogenesis, and the polyclonal human anti-HPA-1a IgG has also showed inhibitory effects on vessel tube formation using human umbilical vein endothelial cells (HUVECs) (107). Importantly, this observation was further supported by elegant work done in human FNAIT patients, which demonstrated that anti-endothelial αVβ3 antibodies are associated with ICH in human patients (165). These findings may give rise to new clinical interventions such as better detection of anti-αVβ3 antibodies in expectant mothers with higher risks of developing ICH in their fetuses.
It seems that there are significant mechanistic differences in hemostasis between fetuses and adults since combined deficiencies of both platelets and coagulation do not lead to bleeding in fetuses (58). If this is true for humans, platelet transfusion to fetus may therefore be a less efficient therapy to control fetal bleeding. This question should be addressed in the near future.
Disruption of placental structure and function
Placental pathology and its role in miscarriage have not been as extensively studied as bleeding diatheses in FNAIT (106). Healthy placenta development involves blastocyst implantation followed by spiral artery development (166,167). These crucial processes involve trophoblast cell migration which is tightly controlled by a subset of NK cells (i.e., decidual and uterine NK cells; d/µNK cells) (168,169). Maternal alloantibodies in FNAIT targeting integrin β3, expressed with αV or αIIb on the surface of trophoblasts, may significantly impair trophoblast function, and lead to life-threatening complications as IUGR or miscarriage (106,167,170,171).
Recent discoveries, however, demonstrated that IUGR was perpetuated by the recruitment of pro-inflammatory µNK cells (i.e., upregulation of activating markers NKp46, FcγRIIIa, perforin release) in mid-gestation (106,172). While it is previously known that perturbation of µNK function results from a variety of self and non-self signals (e.g., IL-17 produced from Th17 cells, LPS), how NK cells are involved in antibody-mediated pathologies such as FNAIT has not been previously well studied. Our findings in the β3 model showed activation of NK cells from a quiescent to cytotoxic state (106).
We observed that pro-inflammatory µNK cells recruited to the placenta in mid-gestation induced apoptosis in trophoblasts that were bound by anti-β3 integrin complexes. NK cells expressing FcγRIIIa receptors interact with the Fc portion of antibodies, become activated and release perforin and other cytotoxic molecules, in a process known as antibody-dependent cell-mediated cytotoxicity (ADCC). Conversely, this process may be inhibited by NK depletion (anti-asialo-GM-1) or inhibition of activating receptors (e.g., NKp46) (106). FNAIT was abrogated through administration of IVIG or anti-FcRn antibodies (55,88,106). These findings have demonstrated yet another non-classical, antibody-mediated mechanism resulting in the pathogenesis of FNAIT (i.e., apoptosis of trophoblasts, impairment of vascular development in placenta, decrease of blood supply to the fetuses, leading to IUGR and miscarriage).
Diagnosis, treatment, and prophylaxis
Diagnosis and prophylaxis screening for FNAIT
The diagnosis of FNAIT is usually made after detecting severe bleeding or low platelet count (<150×109/L) in fetuses or neonates (66). FNAIT is only diagnosed if no other etiology can be identified or if there is a previous history of FNAIT. The relatively more common prevalence of HPA-1a mediated FNAIT has prompted investigation into the benefits of screening women for HPA-1a genotype (141). Both the expectant mother and her partner can be screened to determine an HPA mismatch, however, the results may be inconclusive if the father is heterozygous (i.e., has shared and distinct HPAs from his partner). The HPA status of the fetus can be determined from fetal DNA present in maternal plasma, however this is not a routine laboratory test yet. The mother can also be screened for the presence of the human leukocyte antigen DRB3*0101 allele (173), which may be an indicator for developing alloantibodies against HPA-1a (174,175).
Another method of screening is to detect anti-HPA alloantibodies in the maternal serum at approximately 20 weeks of gestation (4,113,176). The gold standard method to perform this task is the monoclonal antibody immobilization of platelet antigens (MAIPA) assay (177-180). Even with the sensitivity of MAIPA, antibodies may not be detectable at birth in some rare cases of FNAIT (181). For instance, HPA-3 (αIIb/GPIIb) and HPA-15 (CD109) are challenging to detect as their antigen expression varies with the method of platelet storage and preparations (182-184). Furthermore, divalent cation-chelating anticoagulants used during maternal blood collection can potentially attenuate MAIPA recognition of certain alloantibodies, possibly reducing detection of anti-αIIb-mediated FNAIT (67,185-187). Therefore, MAIPA may not be sensitive enough to detect all pathological maternal antibodies in FNAIT. In such cases it is possible to detect antibodies with a different assay such as surface plasmon resonance (188), however this is not commonly practiced. Most recently, the new technique of self-assembling monolayer coupled to platelet receptors has emerged with great potential to increase the sensitivity and specificity for anti-platelet antibody detection (189). While some studies suggest that detection of maternal anti-HPA alloantibodies is predictive of developing FNAIT (179), other studies don’t find a correlation (104,190). Currently, there is no basis for screening as the cost-benefit analysis is unfavorable and there may be an increased risk of overdiagnosis and unnecessary intervention.
Prophylaxis: antibody mediated immune suppression (AMIS)
FNAIT can be considered the platelet analogue of haemolytic disease of the fetus and newborn (HDFN), another antibody mediated pregnancy complication. HDFN is treated using anti-D prophylaxis, a type of AMIS, which works by preventing a maternal immune response via antibody administration. Due to the prevalence of HPA-1a mediated FNAIT, the potential clinical benefit of anti-HPA-1a prophylaxis was investigated using the β3-/- mouse model (191). The study of this preventative strategy was a proof-of-concept that AMIS could also be an effective prophylactic treatment in a β3-mediated model of FNAIT (191). These studies have led to an initiative to collect plasma to manufacture an anti-HPA-1a IgG product for the purpose of testing the efficacy of HPA-1a prophylaxis (191). The PROFNAIT project (http://www.profnait.eu/profnait-project/project-funding/) was a European union funded project from 2012–2018 to develop anti-HPA-1a immunoglobulin for prophylaxis, which has orphan status in Europe and the USA and clinical studies are in progress.
Interestingly, it seems that the AMIS is not antigen but platelet specific since anti-β3 antibody can also decrease the immune response against platelet GPIbα (191). This broadens its application for other alloantigens in FNAIT. It is currently unknown whether a monoclonal antibody or mixed monoclonal antibodies can be used to replace the anti-HPA-1a immunoglobulin collected from pregnant women with FNAIT (187,192). If so, the limited resource of anti-HPA-1a antisera can be overcome.
Antenatal treatment
In multigravida with a history of FNAIT, or if anti-platelet alloantibodies are detected, antenatal treatment of FNAIT becomes possible. Several studies have shown a fetal benefit to administering IVIG to the mother during gestation (193,194), however efficacy is still controversial (194-199). Cases mediated by different platelet antigens may be more or less responsive to IVIG therapy, therefore, further study is required to determine responders versus non-responders, and standardize treatment (115). Steroid treatments, such as prednisone may also be administered during pregnancy however the efficacy and potentially harmful side effects must be carefully considered. In a study comparing the usage of single and combination IVIG and prednisone drug therapies, a favorable response was observed in those treated with IVIG alone (196) whereas combination therapy of IVIG and prednisone was reported to have more unfavorable side effects (200). It is now generally accepted to stratify patients based on relative risk of developing FNAIT as judged by maternal history, and propose treatments accordingly (201).
Postnatal treatment
If FNAIT is diagnosed in the neonate, treatment must begin promptly. Some guidelines call for the use of intravenous immunoglobulin (IVIG) therapy, a blood product made from pooled IgG from >1,000 donors. If the infant is severely thrombocytopenic, platelet transfusion should be considered. Despite a lack of standardized trials, a lower threshold of 30×109/L is normally used to indicate treatment for neonates (i.e., immediate transfusion with random donor platelets, if antigen negative platelets are not available) (66,113,202-204). Thrombopoietin (TPO), a primarily liver-generated hormone that stimulates the production of platelets (205,206), and TPO mimetics are useful treatments in ITP, however it is unknown whether these drugs may be of some value in FNAIT to increase neonatal platelet production.
The novel and potential new therapies for FNAIT
Recombinant anti-HPA-1a
A newly developed human recombinant anti-HPA-1a antibody (B2G1) (207), competes with the maternal alloantibodies for binding to fetal HPA-1a positive platelets and may be a potential future therapy. The antibody Fc region of B2G1 is modified to prevent binding to Fcγ receptors on macrophages and subsequent phagocytosis. Promising preclinical studies show that in the presence of maternal and B2G1 antibodies the platelets lasted 3 times longer in circulation. Another anti-HPA-1a antibody, known as 26.4, was developed with a higher affinity for HPA-1a+ platelets than B2GI (208) and works to opsonize HPA-1a+ platelets for destruction. In addition, several monoclonal antibodies developed by our group have also been demonstrated to be able to block pathogenic anti-HPA-1a binding to platelets (187,192). Future clinical trials should be performed to confirm their therapeutic potential.
Anti-FcRn therapy
FcRn is the Fc receptor responsible for extending the life of circulating IgG, transporting maternal IgG from milk across neonatal gut epithelium, and placental transport of maternal IgG from maternal circulation to fetal circulation (209). IgG naturally binds FcRn at low pH and is released at physiological pH (210). The efficacy of anti-FcRn monoclonal antibodies in ameliorating FNAIT symptoms (e.g., low platelet count) have been applied in several mouse models with promising results (55,105-107). As previously mentioned, anti-FcRn is thought to block fetal FcRn and prevent the transport of maternal IgG into fetal circulation. Although monoclonal antibodies are frequently used as therapeutics, there is still a lot more work that needs to be done to assess the safety and efficacy of anti-FcRn as a therapeutic before its use in a clinical setting.
Anti-NK cell therapy
Our group recently demonstrated that uterine resident NK cells in anti-β3-mediated FNAIT can switch from a quiescent non-cytotoxic phenotype to an activated cytotoxic phenotype (106). Monoclonal antibodies that were used to deplete NK cells or block activating receptors (NKp46 or FcγRIIIa), proved to be an effective in utero treatment shown in our FNAIT mouse models, ameliorating much of the miscarriage and bleeding associated with anti-β3-mediated FNAIT. Although still in its early stages, these observations open the door to other therapeutic targets on NK cells and potentially other placental resident immune cells (59). Again, the efficacy and safety should be evaluated in future clinical trials.
Conclusions and future perspectives
This article summarizes the progress that has been made in FNAIT pathogenesis and treatment. We have identified several “non-classical” immune-mediated and antigen specific mechanisms resulting in potential miscarriage in FNAIT. For instance, alloantibodies against platelet antigens (i.e., β3) can bind a wide range of cells (e.g., endothelial) that further contribute to pathogenesis of FNAIT like ICH (107), than previously known. Integrin β3+ trophoblasts may also be targeted to impair placenta development and contribute to IUGR and miscarriage (106). We have also shown that uterine resident NK cells may switch to a cytotoxic phenotype and impair placental development in FNAIT (106). However, the role of other regulatory cells in the vicinity is unknown. Another non-classical FNAIT pathology includes anti-GPIbα alloantibodies leading to platelet activation, thrombosis and placental dysfunction (55). Collectively, these mechanisms cause distinct pathophysiology leading to miscarriage compared thrombocytopenia alone, and lead to new approaches in modulating treatment. Broadening our understanding of the pathophysiology of FNAIT has fueled the exploration of novel treatments including anti-HPA-1a recombinant antibodies for prophylaxis, anti-FcRn and anti-NK cell therapies. Overall, since there is no standardized protocol for the treatment of FNAIT so far, therein lies great opportunity for potential development of new therapeutics and prophylaxis targeting this life-threatening disease.
Acknowledgments
Funding: This work was supported in part by Canadian Institutes of Health Research (CIHR: MOP 119540, MOP 97918, MOP 68986 and MOP 119551), Canadian Institutes of Health Research Foundation grant (389035), CIHR-Canadian Blood Services Partnership and a grant-in-aid from the Heart and Stroke Foundation of Canada (Ontario). ZY Chen is a recipient of the Fellowship from Department of Laboratory Medicine and Pathobiology, the University of Toronto. BE Oswald is a recipient of the Queen Elizabeth II Graduate Scholarship in Science and Technology. JA Sullivan is a recipient of the Canadian Institutes of Health Research Canadian Graduate Student – Master Award and the Fellowship from Department of Laboratory Medicine and Pathobiology, the University of Toronto.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sentot Santoso) for the series “Platelet Immunology” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: The series “Platelet Immunology” was commissioned by the editorial office without any funding or sponsorship. Integrin β3 anti-PSI monoclonal antibodies are patented in the United States, Canada, and Europe (United States Patent Application No. 12/082686; Canadian Patent application No. 2628900; European Patent Application No. 08153880.3)
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xu XR, Zhang D, Oswald BE, et al. Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit Rev Clin Lab Sci 2016;53:409-30. [Crossref] [PubMed]
- Junt T, Schulze H, Chen Z, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science 2007;317:1767-70. [Crossref] [PubMed]
- Lefrançais E, Ortiz-Munoz G, Caudrillier A, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 2017;544:105-9. [Crossref] [PubMed]
- Michelson AD. 38 - The Clinical Approach to Disorders of Platelet Number and Function. In: Michelson AD. editor. Platelets. Fourth Edition. Cambridge, Massachusetts: Academic Press, 2019:701-5.
- Coller BS. Historical perspective and future directions in platelet research. J Thromb Haemost 2011;9:374-95. [Crossref] [PubMed]
- Murphy AJ, Bijl N, Yvan-Charvet L, et al. Cholesterol efflux in megakaryocyte progenitors suppresses platelet production and thrombocytosis. Nat Med 2013;19:586-94. [Crossref] [PubMed]
- Lindemann S, Kramer B, Seizer P, et al. Platelets, inflammation and atherosclerosis. J Thromb Haemost 2007;5:203-11. [Crossref] [PubMed]
- Italiano JE, Lecine P, Shivdasani RA, et al. Blood Platelets Are Assembled Principally at the Ends of Proplatelet Processes Produced by Differentiated Megakaryocytes. J Cell Biol 1999;147:1299. [Crossref] [PubMed]
- Chatterjee M, Huang Z, Zhang W, et al. Distinct platelet packaging, release, and surface expression of proangiogenic and antiangiogenic factors on different platelet stimuli. Blood 2011;117:3907-11. [Crossref] [PubMed]
- Xu XR, Yousef GM, Ni H. Cancer and platelet crosstalk: opportunities and challenges for aspirin and other antiplatelet agents. Blood 2018;131:1777-89. [Crossref] [PubMed]
- Hess PR, Rawnsley DR, Jakus Z, et al. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. J Clin Invest 2014;124:273-84. [Crossref] [PubMed]
- Osada M, Inoue O, Ding G, et al. Platelet activation receptor CLEC-2 regulates blood/lymphatic vessel separation by inhibiting proliferation, migration, and tube formation of lymphatic endothelial cells. J Biol Chem 2012;287:22241-52. [Crossref] [PubMed]
- Lesurtel M, Graf R, Aleil B, et al. Platelet-derived serotonin mediates liver regeneration. Science 2006;312:104-7. [Crossref] [PubMed]
- Lisman T, Porte RJ. Mechanisms of platelet-mediated liver regeneration. Blood 2016;128:625-9. [Crossref] [PubMed]
- Li C, Li J, Li Y, et al. Crosstalk between Platelets and the Immune System: Old Systems with New Discoveries. Adv Hematol 2012;2012:384685 [Crossref] [PubMed]
- Semple JW, Italiano JE, Freedman J. Platelets and the immune continuum. Nat Rev Immunol 2011;11:264-74. [Crossref] [PubMed]
- Yang H, Lang S, Zhai Z, et al. Fibrinogen is required for maintenance of platelet intracellular and cell-surface P-selectin expression. Blood 2009;114:425-36. [Crossref] [PubMed]
- Thornton P, McColl BW, Greenhalgh A, et al. Platelet interleukin-1alpha drives cerebrovascular inflammation. Blood 2010;115:3632-9. [Crossref] [PubMed]
- Wang Y, Andrews M, Yang Y, et al. Platelets in thrombosis and hemostasis: old topic with new mechanisms. Cardiovasc Hematol Disord Drug Targets 2012;12:126-32. [Crossref] [PubMed]
- Xu XR, Gallant RC, Ni H. Platelets, immune-mediated thrombocytopenias, and fetal hemorrhage. Thromb Res 2016;141:S76-9. [Crossref] [PubMed]
- Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med 2002;346:995-1008. [Crossref] [PubMed]
- Kaplan C, Ni H, Freedman J. 46 - Alloimmune Thrombocytopenia. In: Michelson AD, editor. Platelets (Third Edition). 3rd Edition ed. Waltham: Elsevier Inc., 2013:953-71.
- Wang Y, Gallant RC, Ni H. Extracellular matrix proteins in the regulation of thrombus formation. Curr Opin Hematol 2016;23:280-7. [Crossref] [PubMed]
- Ruggeri ZM. Platelets in atherothrombosis. Nat Med 2002;8:1227-34. [Crossref] [PubMed]
- Lei X, Reheman A, Hou Y, et al. Anfibatide, a novel GPIb complex antagonist, inhibits platelet adhesion and thrombus formation in vitro and in vivo in murine models of thrombosis. Thromb Haemost 2014;111:279-89. [Crossref] [PubMed]
- Nieswandt B, Brakebusch C, Bergmeier W, et al. Glycoprotein VI but not alpha2beta1 integrin is essential for platelet interaction with collagen. EMBO J 2001;20:2120-30. [Crossref] [PubMed]
- Yao Y, Chen Y, Adili R, et al. Plant-based Food Cyanidin-3-Glucoside Modulates Human Platelet Glycoprotein VI Signaling and Inhibits Platelet Activation and Thrombus Formation. J Nutr 2017;147:1917-25. [Crossref] [PubMed]
- Ni H, Ramakrishnan V, Ruggeri ZM, et al. Increased thrombogenesis and embolus formation in mice lacking glycoprotein V. Blood 2001;98:368-73. [Crossref] [PubMed]
- Gui T, Reheman A, Funkhouser WK, et al. In vivo response to vascular injury in the absence of factor IX: examination in factor IX knockout mice. Thromb Res 2007;121:225-34. [Crossref] [PubMed]
- Gui T, Reheman A, Ni H, et al. Abnormal hemostasis in a knock-in mouse carrying a variant of factor IX with impaired binding to collagen type IV. J Thromb Haemost 2009;7:1843-51. [Crossref] [PubMed]
- Ni H, Freedman J. Platelets in hemostasis and thrombosis: role of integrins and their ligands. Transfus Apher Sci 2003;28:257-64. [Crossref] [PubMed]
- Ya F, Xu XR, Shi Y, et al. Coenzyme Q10 Upregulates Platelet cAMP/PKA Pathway and Attenuates Integrin alphaIIbbeta3 Signaling and Thrombus Growth. Mol Nutr Food Res 2019;e1900662 [Crossref] [PubMed]
- Shen B, Zhao X, O'Brien KA, et al. A directional switch of integrin signalling and a new anti-thrombotic strategy. Nature 2013;503:131-5. [Crossref] [PubMed]
- Wilkins JA, Li A, Ni H, et al. Control of integrin function localization of stimulatory epitopes. J Biol Chem 1996;271:3046-51. [Crossref] [PubMed]
- Ni H, Wilkins JA. Localisation of a novel adhesion blocking epitope on the human β1 integrin chain. Cell Adhes Commun 1998;5:257-71. [Crossref] [PubMed]
- Ni H, Li A, Simonsen N, et al. Integrin Activation by Dithiothreitol or Mn2+ Induces a Ligand-occupied Conformation and Exposure of a Novel NH2-terminal Regulatory Site on the β1Integrin Chain. J Biol Chem 1998;273:7981-7. [Crossref] [PubMed]
- Brown JH, Volkmann N, Jun G, et al. The crystal structure of modified bovine fibrinogen. Proc Natl Acad Sci U S A 2000;97:85-90. [Crossref] [PubMed]
- Rocco M, Rosano C, Weisel JW, et al. Integrin conformational regulation: uncoupling extension/tail separation from changes in the head region by a multiresolution approach. Structure 2008;16:954-64. [Crossref] [PubMed]
- Hou Y, Carrim N, Wang Y, et al. Platelets in hemostasis and thrombosis: Novel mechanisms of fibrinogen-independent platelet aggregation and fibronectin-mediated protein wave of hemostasis. J Biomed Res 2015;29:437-44. [PubMed]
- Ni H, Denis CV, Subbarao S, et al. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J Clin Invest 2000;106:385-92. [Crossref] [PubMed]
- Reheman A, Yang H, Zhu G, et al. Plasma fibronectin depletion enhances platelet aggregation and thrombus formation in mice lacking fibrinogen and von Willebrand factor. Blood 2009;113:1809-17. [Crossref] [PubMed]
- Yang H, Reheman A, Chen P, et al. Fibrinogen and von Willebrand factor-independent platelet aggregation in vitro and in vivo. J Thromb Haemost 2006;4:2230-7. [Crossref] [PubMed]
- Wang Y, Reheman A, Spring CM, et al. Plasma fibronectin supports hemostasis and regulates thrombosis. J Clin Invest 2014;124:4281-93. [Crossref] [PubMed]
- Xu X, Wu J, Zhai Z, et al. A novel fibrinogen Bbeta chain frameshift mutation in a patient with severe congenital hypofibrinogenaemia. Thromb Haemost 2006;95:931-5. [Crossref] [PubMed]
- Zhai Z, Wu J, Xu X, et al. Fibrinogen controls human platelet fibronectin internalization and cell-surface retention. J Thromb Haemost 2007;5:1740-6. [Crossref] [PubMed]
- Dunne E, Spring CM, Reheman A, et al. Cadherin 6 has a functional role in platelet aggregation and thrombus formation. Arterioscler Thromb Vasc Biol 2012;32:1724-31. [Crossref] [PubMed]
- Reheman A, Tasneem S, Ni H, et al. Mice with deleted multimerin 1 and α-synuclein genes have impaired platelet adhesion and impaired thrombus formation that is corrected by multimerin 1. Thromb Res 2010;125:e177-83. [Crossref] [PubMed]
- Reheman A, Gross P, Yang H, et al. Vitronectin stabilizes thrombi and vessel occlusion but plays a dual role in platelet aggregation. J Thromb Haemost 2005;3:875-83. [Crossref] [PubMed]
- Ni H, Yuen PS, Papalia JM, et al. Plasma fibronectin promotes thrombus growth and stability in injured arterioles. Proc Natl Acad Sci U S A 2003;100:2415-9. [Crossref] [PubMed]
- Wang Y, Gallant RC, Neves MAD, et al. Alpha-Dystroglycan Supports Platelet Aggregation and Thrombus Formation. Blood 2019;134:11. [PubMed]
- Eltringham-Smith LJ, Yu R, Qadri SM, et al. Prothrombin, alone or in complex concentrates or plasma, reduces bleeding in a mouse model of blood exchange-induced coagulopathy. Sci Rep 2019;9:13029. [Crossref] [PubMed]
- Eltringham-Smith LJ, Lei X, Reheman A, et al. The fibrinogen but not the Factor VIII content of transfused plasma determines its effectiveness at reducing bleeding in coagulopathic mice. Transfusion 2015;55:1040-50. [Crossref] [PubMed]
- Roberts HR, Hoffman M, Monroe DM. A cell-based model of thrombin generation. Semin Thromb Hemost 2006;32:32-8. [Crossref] [PubMed]
- Rand ML, Wang H, Bang KWA, et al. Phosphatidylserine exposure and other apoptotic-like events in Bernard-Soulier syndrome platelets. American Journal of Hematology 2010;85:584-92. [Crossref] [PubMed]
- Li C, Piran S, Chen P, et al. The maternal immune response to fetal platelet GPIbα causes frequent miscarriage in mice that can be prevented by intravenous IgG and anti-FcRn therapies. J Clin Invest 2011;121:4537-47. [Crossref] [PubMed]
- Ni H. Unveiling the new face of fibronectin in thrombosis and hemostasis. J Thromb Haemost 2006;4:940-2. [Crossref] [PubMed]
- Ni H, Papalia JM, Degen JL, et al. Control of thrombus embolization and fibronectin internalization by integrin αIIbβ3 engagement of the fibrinogen γ chain. Blood 2003;102:3609-14. [Crossref] [PubMed]
- Palumbo JS, Zogg M, Talmage KE, et al. Role of fibrinogen- and platelet-mediated hemostasis in mouse embryogenesis and reproduction. J Thromb Haemost 2004;2:1368-79. [Crossref] [PubMed]
- Yougbaré I, Zdravic D, Ni H. Fetal and neonatal alloimmune thrombocytopenia: Novel mechanisms of miscarriage learned from placental pathology in animal models. J Pediatr Pediatr Med 2018;2:28-33. [Crossref]
- Cines DB, Bussel JB, Liebman HA, et al. The ITP syndrome: pathogenic and clinical diversity. Blood 2009;113:6511-21. [Crossref] [PubMed]
- Despotovic JM, Bussel JB. 39 - Immune Thrombocytopenia (ITP). In: Michelson AD. editor. Platelets. Fourth Edition. Cambridge, Massachusetts: Academic Press, 2019:707-24.
- Lambert MP, Poncz M. 46 - Inherited Thrombocytopenias. In: Michelson AD. editor. Platelets Fourth Edition. Cambridge, Massachusetts: Academic Press, 2019:849-61.
- Balduini CL, Melazzini F, Pecci A. Inherited thrombocytopenias-recent advances in clinical and molecular aspects. Platelets 2017;28:3-13. [Crossref] [PubMed]
- Kuter DJ. Managing thrombocytopenia associated with cancer chemotherapy. Oncology (Williston Park) 2015;29:282-94. [PubMed]
- Hitron A, Steinke D, Sutphin S, et al. Incidence and risk factors of clinically significant chemotherapy-induced thrombocytopenia in patients with solid tumors. J Oncol Pharm Pract 2011;17:312-9. [Crossref] [PubMed]
- Kaplan C, Bertrand G, Ni H. 45 - Alloimmune Thrombocytopenia. In: Michelson ADBTP. editor. Platelets. Fourth Edition. Cambridge, Massachusetts: Academic Press, 2019:833-48.
- Li J, Sullivan JA, Ni H. Pathophysiology of immune thrombocytopenia. Curr Opin Hematol 2018;25:373-81. [Crossref] [PubMed]
- Neunert C, Noroozi N, Norman G, et al. Severe bleeding events in adults and children with primary immune thrombocytopenia: a systematic review. J Thromb Haemost 2015;13:457-64. [Crossref] [PubMed]
- Crow AR, Lazarus AH. Role of Fcγ receptors in the pathogenesis and treatment of idiopathic thrombocytopenic purpura. J Pediatr Hematol Oncol 2003;25:S14-8. [Crossref] [PubMed]
- Aslam R, Kapur R, Segel GB, et al. The spleen dictates platelet destruction, anti-platelet antibody production, and lymphocyte distribution patterns in a murine model of immune thrombocytopenia. Exp Hematol 2016;44:924-30.e1. [Crossref] [PubMed]
- Nieswandt B, Bergmeier W, Rackebrandt K, et al. Identification of critical antigen-specific mechanisms in the development of immune thrombocytopenic purpura in mice. Blood 2000;96:2520-7. [Crossref] [PubMed]
- Webster ML, Sayeh E, Crow M, et al. Relative efficacy of intravenous immunoglobulin G in ameliorating thrombocytopenia induced by antiplatelet GPIIbIIIa versus GPIb$α$ antibodies. Blood 2006;108:943-6. [Crossref] [PubMed]
- Webster ML, Zhu G, Li Y, et al. Fc-independent phagocytosis: implications for intravenous IgG therapy in immune thrombocytopenia. Cardiovasc Hematol Disord Drug Targets 2008;8:278-82. [Crossref] [PubMed]
- Olsson B, Andersson PO, Jernås M, et al. T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med 2003;9:1123. [Crossref] [PubMed]
- Chow L, Aslam R, Speck ER, et al. A murine model of severe immune thrombocytopenia is induced by antibody-and CD8+ T cell–mediated responses that are differentially sensitive to therapy. Blood 2010;115:1247-53. [Crossref] [PubMed]
- Qiu J, Liu X, Li X, et al. CD8+ T cells induce platelet clearance in the liver via platelet desialylation in immune thrombocytopenia. Sci Rep 2016;6:27445. [Crossref] [PubMed]
- Ma L, Simpson E, Li J, et al. CD8+ T cells are predominantly protective and required for effective steroid therapy in murine models of immune thrombocytopenia. Blood 2015;126:247-56. [Crossref] [PubMed]
- Yazdanbakhsh K, Zhong H, Bao W. Immune dysregulation in immune thrombocytopenia. Semin Hematol 2013;50:S63-7. [Crossref] [PubMed]
- van Der Wal DE, Zhu G, Li J, et al. Desialylation: a novel platelet clearance mechanism and a potential new therapeutic target in anti-GPIb antibody mediated thrombocytopenia. Am Soc Hematology 2012;120:265.
- Li J, van der Wal DE, Zhu G, et al. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat Commun 2015;6:7737. [Crossref] [PubMed]
- Quach ME, Dragovich MA, Chen W, et al. Fc-independent immune thrombocytopenia via mechanomolecular signaling in platelets. Blood 2018;131:787-96. [Crossref] [PubMed]
- Li J,, van der Wal DE, Zhu L, et al. Fc-independent phagocytosis: implications for IVIG and other therapies in immune-mediated thrombocytopenia. Cardiovasc Hematol Disord Drug Targets 2013;13:50-8. [Crossref] [PubMed]
- Li C, Chen P, Vadasz B, et al. Co-stimulation with LPS or Poly I:C markedly enhances the anti-platelet immune response and severity of fetal and neonatal alloimmune thrombocytopenia. Thromb Haemost 2013;110:1250-8. [Crossref] [PubMed]
- Nishimoto T, Satoh T, Simpson E, et al. Predominant autoantibody response to GPIb/IX in a regulatory T‐cell‐deficient mouse model for immune thrombocytopenia. J Thromb Haemost 2013;11:369-72. [Crossref] [PubMed]
- Go RS, Johnston KL, Bruden KC. The association between platelet autoantibody specificity and response to intravenous immunoglobulin G in the treatment of patients with immune thrombocytopenia. Haematologica 2007;92:283-4. [Crossref] [PubMed]
- Peng J, Ma SH, Liu J, et al. Association of autoantibody specificity and response to intravenous immunoglobulin G therapy in immune thrombocytopenia: a multicenter cohort study. J Thromb Haemost 2014;12:497-504. [Crossref] [PubMed]
- Crow AR, Song S, Semple JW, et al. IVIg inhibits reticuloendothelial system function and ameliorates murine passive‐immune thrombocytopenia independent of anti‐idiotype reactivity. Br J Haematol 2001;115:679-86. [Crossref] [PubMed]
- Ni H, Chen P, Spring CM, et al. A novel murine model of fetal and neonatal alloimmune thrombocytopenia: Response to intravenous IgG therapy. Blood 2006;107:2976-83. [Crossref] [PubMed]
- Leytin V, Mykhaylov S, Starkey AF, et al. Intravenous immunoglobulin inhibits anti‐glycoprotein IIb‐induced platelet apoptosis in a murine model of immune thrombocytopenia. Br J Haematol 2006;133:78-82. [PubMed]
- Zeng Q, Zhu L, Tao L, et al. Relative efficacy of steroid therapy in immune thrombocytopenia mediated by anti-platelet GPIIbIIIa versus GPIbalpha antibodies. Am J Hematol 2012;87:206-8. [Crossref] [PubMed]
- Li J, Callum JL, Lin Y, et al. Severe platelet desialylation in a patient with glycoprotein Ib/IX antibody-mediated immune thrombocytopenia and fatal pulmonary hemorrhage. Haematologica 2014;99:e61 [Crossref] [PubMed]
- Chen M, Yan R, Zhou K, et al. Akt-mediated platelet apoptosis and its therapeutic implications in immune thrombocytopenia. Proc Natl Acad Sci U S A 2018;115:E10682-91. [Crossref] [PubMed]
- Li Y, Fu J, Ling Y, et al. Sialylation on O-glycans protects platelets from clearance by liver Kupffer cells. Proc Natl Acad Sci U S A 2017;114:8360-5. [Crossref] [PubMed]
- Tao L, Zeng Q, Li J, et al. Platelet desialylation correlates with efficacy of first-line therapies for immune thrombocytopenia. J Hematol Oncol 2017;10:46. [Crossref] [PubMed]
- Jansen AJ, Peng J, Zhao HG, et al. Sialidase inhibition to increase platelet counts: A new treatment option for thrombocytopenia. Am J Hematol 2015;90:E94-5. [Crossref] [PubMed]
- Shao L, Wu Y, Zhou H, et al. Successful treatment with oseltamivir phosphate in a patient with chronic immune thrombocytopenia positive for anti-GPIb/IX autoantibody. Platelets 2015;26:495-7. [Crossref] [PubMed]
- Bigot P, Auffret M, Gautier S, et al. Unexpected platelets elevation in a patient with idiopathic thrombocytopenia treated with oseltamivir for influenza infection. Fundam Clin Pharmacol 2016;30:483-5. [Crossref] [PubMed]
- Revilla N, Corral J, Miñano A, et al. Multirefractory primary immune thrombocytopenia; targeting the decreased sialic acid content. Platelets 2019;30:743-51. [Crossref] [PubMed]
- Curtis BR. Recent progress in understanding the pathogenesis of fetal and neonatal alloimmune thrombocytopenia. Br J Haematol 2015;171:671-82. [Crossref] [PubMed]
- Bussel JB, Primiani A. Fetal and neonatal alloimmune thrombocytopenia: progress and ongoing debates. Blood Rev 2008;22:33-52. [Crossref] [PubMed]
- Vadasz B, Chen P, Yougbaré I, et al. Platelets and platelet alloantigens: Lessons from human patients and animal models of fetal and neonatal alloimmune thrombocytopenia. Genes Dis 2015;2:173-85. [Crossref] [PubMed]
- Zdravic D, Yougbare I, Vadasz B, et al. Fetal and neonatal alloimmune thrombocytopenia. Semin Fetal Neonatal Med 2016;21:19-27. [Crossref] [PubMed]
- Butros LJ, Bussel JB. Intracranial hemorrhage in immune thrombocytopenic purpura: a retrospective analysis. J Pediatr Hematol Oncol 2003;25:660-4. [Crossref] [PubMed]
- Ghevaert C, Campbell K, Walton J, et al. Management and outcome of 200 cases of fetomaternal alloimmune thrombocytopenia. Transfusion 2007;47:901-10. [Crossref] [PubMed]
- Chen P, Li C, Lang S, et al. Animal model of fetal and neonatal immune thrombocytopenia: role of neonatal Fc receptor in the pathogenesis and therapy. Blood 2010;116:3660-8. [Crossref] [PubMed]
- Yougbaré I, Tai WS, Zdravic D, et al. Activated NK cells cause placental dysfunction and miscarriages in fetal alloimmune thrombocytopenia. Nat Commun 2017;8:224. [Crossref] [PubMed]
- Yougbaré I, Lang S, Yang H, et al. Maternal anti-platelet β3 integrins impair angiogenesis and cause intracranial hemorrhage. J Clin Invest 2015;125:1545-56. [Crossref] [PubMed]
- Giovangrandi Y, Daffos F, Kaplan C, et al. Very early intracranial haemorrhage in alloimmune fetal thrombocytopenia. Lancet 1990;336:310. [Crossref] [PubMed]
- de Vries LS, Connell J, Bydder GM, et al. Recurrent intracranial haemorrhages in utero in an infant with alloimmune thrombocytopenia. Case report. Br J Obstet Gynaecol 1988;95:299-302. [Crossref] [PubMed]
- Bussel JB, Zacharoulis S, Kramer K, et al. Clinical and diagnostic comparison of neonatal alloimmune thrombocytopenia to non-immune cases of thrombocytopenia. Pediatr Blood Cancer 2005;45:176-83. [Crossref] [PubMed]
- Hayashi T, Hirayama F. Advances in alloimmune thrombocytopenia: perspectives on current concepts of human platelet antigens, antibody detection strategies, and genotyping. Blood Transfus 2015;13:380. [PubMed]
- Kroll H, Yates J, Santoso S. Immunization against a low‐frequency human platelet alloantigen in fetal alloimmune thrombocytopenia is not a single event: characterization by the combined use of reference DNA and novel allele‐specific cell lines expressing recombinant antigens. Transfusion 2005;45:353-8. [Crossref] [PubMed]
- Peterson JA, McFarland JG, Curtis BR, et al. Neonatal alloimmune thrombocytopenia: pathogenesis, diagnosis and management. Br J Haematol 2013;161:3-14. [Crossref] [PubMed]
- Metcalfe P, Watkins N, Ouwehand W, et al. Nomenclature of human platelet antigens. Vox Sang 2003;85:240-5. [Crossref] [PubMed]
- Regan F, Lees CC, Jones B, et al. Prenatal Management of Pregnancies at Risk of Fetal Neonatal Alloimmune Thrombocytopenia (FNAIT): Scientific Impact Paper No. 61. BJOG 2019;126:e173-85. [Crossref] [PubMed]
- Kjeldsen-Kragh J, Ni H, Skogen B. Towards a prophylactic treatment of HPA-related foetal and neonatal alloimmune thrombocytopenia. Curr Opin Hematol 2012;19:469-74. [Crossref] [PubMed]
- Buitrago L, Rendon A, Liang Y, et al. αIIbβ3 variants defined by next-generation sequencing: predicting variants likely to cause Glanzmann thrombasthenia. Proc Natl Acad Sci U S A 2015;112:E1898-907. [Crossref] [PubMed]
- Silva F, Morais S, Sevivas T, et al. Severe intracranial haemorrhage in neonatal alloimmune thrombocytopenia. BMJ Case Rep 2011; [Crossref] [PubMed]
- Chen P, Issaka Y, Zhu G, et al. Novel Murine Models Of Fetal and Neonatal Alloimmune Thrombocytopenia Established In αIIb Deficient and Human αIIb Transgenic Mice. Blood 2013;122:2314. [Crossref]
- Sullivan J, Oswald BE, Vadasz B, et al. Maternal Anti-αIIb Antibodies Target Fetal Hematopoietic Stem Cells and May Lead to Pancytopenia and Miscarriage in Fnait. Blood 2017;130:233.
- Vadasz B. Pathogenesis of Anti-integrin αIIb-mediated Fetal and Neonatal Alloimmune Thrombocytopenia: Establishment of Novel Murine Models in αIIb Deficient and Human αIIb Transgenic Mice. Toronto, Canada: University of Toronto, 2015.
- Bussel J, Kaplan C, McFarland J. Recommendations for the evaluation and treatment of neonatal autoimmune and alloimmune thrombocytopenia. Thromb Haemost 1991;65:631-4. [PubMed]
- Ohto H, Miura S, Ariga H, et al. The natural history of maternal immunization against foetal platelet alloantigens. Transfus Med 2004;14:399-408. [Crossref] [PubMed]
- Landau M, Rosenberg N. Molecular insight into human platelet antigens: structural and evolutionary conservation analyses offer new perspective to immunogenic disorders. Transfusion 2011;51:558-69. [Crossref] [PubMed]
- Wu G, Zhou Y, Li L, et al. Platelet immunology in China: research and clinical applications. Transfus Med Rev 2017;31:118-25. [Crossref] [PubMed]
- Xu X, Santoso S. Role of CD 36 in immune‐mediated thrombocytopenia in Asian populations. VOXS 2018;13:317-22. [Crossref]
- Ni H. The platelet “sugar high” in diabetes. Blood 2012;119:5949-51. [Crossref] [PubMed]
- Podrez EA, Byzova TV, Febbraio M, et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med 2007;13:1086. [Crossref] [PubMed]
- Pascual G, Avgustinova A, Mejetta S, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 2017;541:41. [Crossref] [PubMed]
- Banesh S, Trivedi V. Therapeutic potentials of Scavenger receptor CD36 mediated innate immune responses against infectious and non-infectious diseases. Curr Drug Discov Technol 2019; [Crossref] [PubMed]
- Xu X, Ye X, Xia W, et al. Studies on CD36 deficiency in South China: two cases demonstrating the clinical impact of anti-CD36 antibodies. Thromb Haemost 2013;110:1199-206. [Crossref] [PubMed]
- Lin M, Xu X, Lee HL, et al. Fetal/neonatal alloimmune thrombocytopenia due to anti‐CD36 antibodies: antibody evaluations by CD36‐transfected cell lines. Transfusion 2018;58:189-95. [Crossref] [PubMed]
- Curtis BR, Ali S, Glazier AM, et al. Isoimmunization against CD36 (glycoprotein IV): description of four cases of neonatal isoimmune thrombocytopenia and brief review of the literature. Transfusion 2002;42:1173-9. [Crossref] [PubMed]
- Kagan KO, Hoopmann M, Kozlowski P. Assessment of Foetal DNA in Maternal Blood – A Useful Tool in the Hands of Prenatal Specialists. Geburtshilfe Frauenheilkd 2012;72:998-1003. [Crossref] [PubMed]
- Rafi I, Chitty L. Cell-free fetal DNA and non-invasive prenatal diagnosis. Br J Gen Pract 2009;59:e146-8. [Crossref] [PubMed]
- Hyett JA, Gardener G, Stojilkovic-Mikic T, et al. Reduction in diagnostic and therapeutic interventions by non-invasive determination of fetal sex in early pregnancy. Prenat Diagn 2005;25:1111-6. [Crossref] [PubMed]
- Yang Y, Todt JC, Svinarich DM, et al. Human trophoblast cell adhesion to extracellular matrix protein, entactin. Am J Reprod Immunol 1996;36:25-32. [Crossref] [PubMed]
- Kumpel BM, Sibley K, Jackson DJ, et al. Ultrastructural localization of glycoprotein IIIa (GPIIIa, beta 3 integrin) on placental syncytiotrophoblast microvilli: implications for platelet alloimmunization during pregnancy. Transfusion 2008;48:2077-86. [Crossref] [PubMed]
- Snir A, Brenner B, Paz B, et al. Presence of Integrin alpha(IIb)beta 3 in early gestation human trophoblasts: possible involvement of fibrin as a matrix ligand. Thromb Res 2010;125:253-6. [Crossref] [PubMed]
- Kjeldsen-Kragh J, Killie MK, Tomter G, et al. A screening and intervention program aimed to reduce mortality and serious morbidity associated with severe neonatal alloimmune thrombocytopenia. Blood 2007;110:833-9. [Crossref] [PubMed]
- Kjeldsen-Kragh J, Titze TL, Lie BA, et al. HLA-DRB3*01:01 exhibits a dose-dependent impact on HPA-1a antibody levels in HPA-1a-immunized women. Blood Adv 2019;3:945-51. [Crossref] [PubMed]
- Tiller H, Killie MK, Husebekk A, et al. Platelet antibodies and fetal growth: maternal antibodies against fetal platelet antigen 1a are strongly associated with reduced birthweight in boys. Acta Obstet Gynecol Scand 2012;91:79-86. [Crossref] [PubMed]
- Simister NE. Placental transport of immunoglobulin G. Vaccine 2003;21:3365-9. [Crossref] [PubMed]
- Saji F, Samejima Y, Kamiura S, et al. Dynamics of immunoglobulins at the feto-maternal interface. Rev Reprod 1999;4:81-9. [Crossref] [PubMed]
- Palmeira P, Quinello C, Silveira-Lessa AL, et al. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012;2012:985646 [Crossref] [PubMed]
- Lakhdari O, Yamamura A, Hernandez GE, et al. Differential Immune Activation in Fetal Macrophage Populations. Sci Rep 2019;9:7677. [Crossref] [PubMed]
- Hoffmeister KM, Falet H. Platelet clearance by the hepatic Ashwell-Morrell receptor: mechanisms and biological significance. Thromb Res 2016;141:S68-72. [Crossref] [PubMed]
- Zhang F, Chu X, Wang L, et al. Cell-mediated lysis of autologous platelets in chronic idiopathic thrombocytopenic purpura. Eur J Haematol 2006;76:427-31. [Crossref] [PubMed]
- Goldman M, Trudel E, Richard L, et al. Neonatal alloimmune thrombocytopenia due to anti-HPA-2b (anti-Koa). Immunohematology 2003;19:43-6. [PubMed]
- Schmaier AH. Are maternal antiplatelet antibodies a prothrombotic condition leading to miscarriage? J Clin Invest 2011;121:4241-3. [Crossref] [PubMed]
- Jeve YB, Davies W. Evidence-based management of recurrent miscarriages. J Hum Reprod Sci 2014;7:159. [Crossref] [PubMed]
- El Hachem H, Crepaux V, May-Panloup P, et al. Recurrent pregnancy loss: current perspectives. Int J Womens Health 2017;9:331. [Crossref] [PubMed]
- Bussel JB, Zabusky MR, Berkowitz RL, et al. Fetal Alloimmune Thrombocytopenia. N Engl J Med 1997;337:22-6. [Crossref] [PubMed]
- Shivdasani RA, Rosenblatt MF, Zucker-Franklin D, et al. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell 1995;81:695-704. [Crossref] [PubMed]
- Suh TT, Holmback K, Jensen NJ, et al. Resolution of spontaneous bleeding events but failure of pregnancy in fibrinogen-deficient mice. Genes Dev 1995;9:2020-33. [Crossref] [PubMed]
- Brooks PC. Clark Ra, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 1994;264:569-71. [Crossref] [PubMed]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002;110:673-87. [Crossref] [PubMed]
- Yang H, Chen P, Boyd SR, et al. Impaired angiogenesis and enhanced intracranial vascular apoptosis in a murine model of fetal and neonatal alloimmune thrombocytopenia (fnaitp). J Thromb Haemost 2007;5 Suppl 2:O-M-084.
- Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature 2005;438:954. [Crossref] [PubMed]
- Liu J, Fraser SD, Faloon PW, et al. A βPix–Pak2a signaling pathway regulates cerebral vascular stability in zebrafish. Proc Natl Acad Sci U S A 2007;104:13990-5. [Crossref] [PubMed]
- Ballabh P, Xu H, Hu F, et al. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med 2007;13:477. [Crossref] [PubMed]
- Bader BL, Rayburn H, Crowley D, et al. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all αv integrins. Cell 1998;95:507-19. [Crossref] [PubMed]
- McCarty JH, Lacy-Hulbert A, Charest A, et al. Selective ablation of αv integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development 2005;132:165-76. [Crossref] [PubMed]
- Yougbare I, Zdravic D, Ni H. Angiogenesis and bleeding disorders in FNAIT. Oncotarget 2015;6:15724-5. [Crossref] [PubMed]
- Santoso S, Wihadmadyatami H, Bakchoul T, et al. Antiendothelial αvβ3 antibodies are a major cause of intracranial bleeding in fetal/neonatal alloimmune thrombocytopenia. Arterioscler Thromb Vasc Biol 2016;36:1517-24. [Crossref] [PubMed]
- Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet 2001;2:538. [Crossref] [PubMed]
- Rout UK, Wang J, Paria BC, et al. α5β1, αVβ3 and the platelet-associated integrin αIIbβ3 coordinately regulate adhesion and migration of differentiating mouse trophoblast cells. Dev Biol 2004;268:135-51. [Crossref] [PubMed]
- Fu B, Li X, Sun R, et al. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal–fetal interface. Proc Natl Acad Sci U S A 2013;110:E231-40. [Crossref] [PubMed]
- Freitag N, Zwier M, Barrientos G, et al. Influence of relative NK–DC abundance on placentation and its relation to epigenetic programming in the offspring. Cell Death Dis 2014;5:e1392 [Crossref] [PubMed]
- Giuliani E, Parkin KL, Lessey BA, et al. Characterization of uterine NK cells in women with infertility or recurrent pregnancy loss and associated endometriosis. Am J Reprod Immunol 2014;72:262-9. [Crossref] [PubMed]
- Farghali MM, El-kholy ALG, Swidan KH, et al. Relationship between uterine natural killer cells and unexplained repeated miscarriage. J Turk Ger Gynecol Assoc 2015;16:214. [Crossref] [PubMed]
- Yougbare I, Tai W, Zdravic D, et al. Pathology of placenta in fetal and neonatal immune thrombocytopenia: roles of TH17 immune responses, anti-platelet antibodies and angiogenic factors: OR031. J Thromb Haemost 2015;13:105.
- Mikkola HKA, Fujiwara Y, Schlaeger TM, et al. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood 2003;101:508-16. [Crossref] [PubMed]
- Williamson LM, Hackett G, Rennie J, et al. The Natural History of Fetomaternal Alloimmunization to the Platelet-Specific Antigen HPA-1a (PlA1, Zwa) as Determined by Antenatal Screening. Blood 1998;92:2280-7. [Crossref] [PubMed]
- Gruel Y, Boizard B, Daffos F, et al. Determination of platelet antigens and glycoproteins in the human fetus. Blood 1986;68:488-92. [Crossref] [PubMed]
- Chakravorty S, Roberts I. How I manage neonatal thrombocytopenia. Br J Haematol 2012;156:155-62. [Crossref] [PubMed]
- Kiefel V, Santoso S, Weisheit M, et al. Monoclonal antibody--specific immobilization of platelet antigens (MAIPA): a new tool for the identification of platelet-reactive antibodies. Blood 1987;70:1722-6. [Crossref] [PubMed]
- Campbell K, Rishi K, Howkins G, et al. A modified rapid monoclonal antibody-specific immobilization of platelet antigen assay for the detection of human platelet antigen (HPA) antibodies: a multicentre evaluation. Vox Sang 2007;93:289-97. [PubMed]
- Bessos H, Killie MK, Matviyenko M, et al. Direct comparison between two quantitative assays in the measurement of maternal anti-HPA-1a antibody in neonatal alloimmune thrombocytopenia (NAIT). Transfus Apher Sci 2008;39:221-7. [Crossref] [PubMed]
- Killie MK, Salma W, Bertelsen E, et al. Quantitative MAIPA: Comparison of different MAIPA protocols. Transfus Apher Sci 2010;43:149-54. [Crossref] [PubMed]
- Killie MK, Husebekk A, Kjeldsen-Kragh J, et al. A prospective study of maternal anti-HPA 1a antibody level as a potential predictor of alloimmune thrombocytopenia in the newborn. Haematologica 2008;93:870-7. [Crossref] [PubMed]
- Romo GM, Dong J-F, Schade AJ, et al. The Glycoprotein Ib-IX-V Complex Is a Platelet Counterreceptor for P-Selectin. J Exp Med 1999;190:803-14. [Crossref] [PubMed]
- Simon DI, Chen Z, Xu H, et al. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J Exp Med 2000;192:193-204. [Crossref] [PubMed]
- Baglia FA, Badellino KO, Li CQ, et al. Factor XI binding to the platelet glycoprotein Ib-IX-V complex promotes factor XI activation by thrombin. J Biol Chem 2002;277:1662-8. [Crossref] [PubMed]
- O'Neill S, Robinson A, Deering A, et al. The platelet integrin αIIbβ3 has an endogenous thiol isomerase activity. J Biol Chem 2000;275:36984-90. [Crossref] [PubMed]
- Stratton TW, Zhu G, Wang Y, et al. Targeting β3 Integrin Psi Domain Inhibits Both Platelet Aggregation and Blood Coagulation: Two Birds with One Stone. Blood 2018;132:1246. [Crossref]
- Zhu G, Zhang Q, Reddy EC, et al. The integrin PSI domain has an endogenous thiol isomerase function and is a novel target for antiplatelet therapy. Blood 2017;129:1840-54. [Crossref] [PubMed]
- Socher I, Andrei-Selmer C, Bein G, et al. Low-avidity HPA-1a alloantibodies in severe neonatal alloimmune thrombocytopenia are detectable with surface plasmon resonance technology. Transfusion 2009;49:943-52. [Crossref] [PubMed]
- Neves MAD, Wang Y, Zhu G, et al. Novel Integrin αIIbβ3 and GPIbα Coatings that Feature Anti-fouling Properties for Platelet Research and Clinical Diagnostics International Society on Thrombosis and Haemostasis (ISTH) 2017 Congress; 2017; Berlin, Germany.
- Turner ML, Bessos H, Fagge T, et al. Prospective epidemiologic study of the outcome and cost-effectiveness of antenatal screening to detect neonatal alloimmune thrombocytopenia due to anti-HPA-1a. Transfusion 2005;45:1945-56. [Crossref] [PubMed]
- Tiller H, Killie MK, Chen P, et al. Toward a prophylaxis against fetal and neonatal alloimmune thrombocytopenia: induction of antibody-mediated immune suppression and prevention of severe clinical complications in a murine model. Transfusion 2012;52:1446-57. [Crossref] [PubMed]
- Zhi H, Ahlen MT, Thinn AMM, et al. High-resolution mapping of the polyclonal immune response to the human platelet alloantigen HPA-1a (Pl(A1)). Blood Adv 2018;2:3001-11. [Crossref] [PubMed]
- Bertrand G, Drame M, Martageix C, et al. Prediction of the fetal status in noninvasive management of alloimmune thrombocytopenia. Blood 2011;117:3209-13. [Crossref] [PubMed]
- Bussel JB, Berkowitz RL, McFarland JG, et al. Antenatal treatment of neonatal alloimmune thrombocytopenia. N Engl J Med 1988;319:1374-8. [Crossref] [PubMed]
- Bowman J, Harman C, Mentigolou S, et al. Intravenous fetal transfusion of immunoglobulin for alloimmune thrombocytopenia. Lancet 1992;340:1034-5. [Crossref] [PubMed]
- Kaplan C, Murphy MF, Kroll H, et al. Feto-maternal alloimmune thrombocytopenia: antenatal therapy with IvIgG and steroids — more questions than answers. Br J Haematol 1998;100:62-5. [Crossref] [PubMed]
- Nicolini U, Tannirandorn Y, Gonzalez P, et al. Continuing controversy in alloimmune thrombocytopenia: Fetal hyperimmunoglobulinemia fails to prevent thrombocytopenia. Am J Obstet Gynecol 1990;163:1144-6. [Crossref] [PubMed]
- Urbaniak SJ, Duncan JI, Armstrong-Fisher SS, et al. Variable inhibition of placental IgG transfer in vitro with commercial IVgG preparations. Br J Haematol 1999;107:815-7. [Crossref] [PubMed]
- Kjeldsen-Kragh J, Husebekk A, Killie MK, et al. The pathophysiology of FNAIT cannot be deduced from highly selected retrospective data. Blood 2011;118:2638-9. [Crossref] [PubMed]
- Berkowitz RL, Lesser ML, McFarland JG, et al. Antepartum treatment without early cordocentesis for standard-risk alloimmune thrombocytopenia: a randomized controlled trial. Obstet Gynecol 2007;110:249-55. [Crossref] [PubMed]
- Pacheco LD, Berkowitz RL, Moise KJ, et al. Fetal and neonatal alloimmune thrombocytopenia: a management algorithm based on risk stratification. Obstet Gynecol 2011;118:1157-63. [Crossref] [PubMed]
- Blanchette VS, Johnson J, Rand M. The management of alloimmune neonatal thrombocytopenia. Baillieres Best Pract Res Clin Haematol 2000;13:365-90. [Crossref] [PubMed]
- Bertrand G, Kaplan C. How do we treat fetal and neonatal alloimmune thrombocytopenia? Transfusion 2014;54:1698-703. [Crossref] [PubMed]
- Rebulla P. Platelet transfusion trigger in difficult patients. Transfus Clin Biol 2001;8:249-54. [Crossref] [PubMed]
- Xu M, Li J, Neves MAD, et al. GPIbalpha is required for platelet-mediated hepatic thrombopoietin generation. Blood 2018;132:622-34. [Crossref] [PubMed]
- Kuter DJ. The biology of thrombopoietin and thrombopoietin receptor agonists. Int J Hematol 2013;98:10-23. [Crossref] [PubMed]
- Ghevaert C, Herbert N, Hawkins L, et al. Recombinant HPA-1a antibody therapy for treatment of fetomaternal alloimmune thrombocytopenia: proof of principle in human volunteers. Blood 2013;122:313-20. [Crossref] [PubMed]
- Eksteen M, Tiller H, Averina M, et al. Characterization of a human platelet antigen-1a-specific monoclonal antibody derived from a B cell from a woman alloimmunized in pregnancy. J Immunol 2015;194:5751-60. [Crossref] [PubMed]
- Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007;7:715. [Crossref] [PubMed]
- Raghavan M, Bonagura VR, Morrison SL, et al. Analysis of the pH dependence of the neonatal Fc receptor/immunoglobulin G interaction using antibody and receptor variants. Biochemistry 1995;34:14649-57. [Crossref] [PubMed]
Cite this article as: Chen ZY, Oswald BE, Sullivan JA, Dahmani FZ, Pasman Y, Liu Z, Chen P, Ni H. Platelet physiology and immunology: pathogenesis and treatment of classical and non-classical fetal and neonatal alloimmune thrombocytopenia. Ann Blood 2019;4:29.


