A case of unfractionated heparin-induced thrombocytopenia during the treatment of a gastric stromal tumor
Introduction
Heparin-induced thrombocytopenia (HIT), which can be life-threatening, is an adverse effect of heparin caused by platelet activation induced by IgG-PF4-heparin immune complexes (1). HIT occurs in 1–3% of heparin-treated patients and is divided into two types: HIT I and HIT II. HIT I is non-immune-mediated and can usually be disregarded because it is self-limiting, and its clinical course is mild. HIT II is defined as a decrease in platelet count caused by an immune response (<150 g/L or over 50%) occurring 5–15 days after the use of unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), or fondaparinux; only a few cases of HIT II have been described (2).
In standard practice, clinical assessments and enzymatic immunoassays (IgG antibodies) are used to diagnose HIT (3). The clinical information that was used in this study to define the 4Ts status (4) was obtained by a physician investigator experienced in HIT diagnosis. This individual assessed the timing of platelet count drop, baseline platelet count, degree of thrombocytopenia, presence of thrombosis, and bleeding and presence of additional diseases; each element is scored from 0 to 2 (4). Scores above 4 indicated that the patient was at high risk of HIT. Here, we report one case, which presented with thrombocytopenia induced by heparin and a positive PF4/heparin antibody test in vitro.
Case presentation
A 37-year-old male suffering from a gastric stromal tumor and liver metastasis sought treatment. The patient had a history of hepatitis B virus infection. The patient developed anemia while undergoing chemotherapy. His platelet count was relatively low (5 g/L); however, his red blood cell and white blood cell counts were close to the normal range. He had been administered cephalosporin antibiotics to fight infection. The patient underwent hemodialysis once a week, during which UFH 2,500 U was used to flush the intravascular catheter. Anticoagulation was performed in vitro using a soak pipe filter.
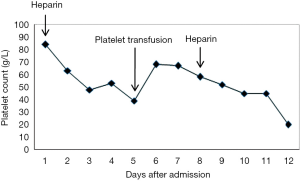
The platelet count decreased to 5 g/L after administration of cross-match compatible platelets in the intensive care unit (ICU). He experienced an intracerebral hemorrhage after administration of UFH to flush the intravascular for a second time. Based on the patient’s medical history, the platelet count decreased after administration of UFH and increased after platelet transfusion, and then decreased after the second administration of UFH (Figure 1). According to the clinical information and the 4T scoring system, the 4T score was 5 (exposed to heparin within the last 30 days, the timing from inclusion to a drop in platelet count was ≤1, platelet count decreased by >50%, minimum platelet count was ≥20 g/L, and thrombocytopenia. Based on these findings, the pretest probability for HIT was high.
Subsequent laboratory testing
To test the patient’s platelet antibodies, we treated the blood samples with an anti-coagulant ethylenediaminetetraacetic acid (EDTA), anti-PF4/heparin IgG was positive (OD =1.205), and the concentration level was 19.957 U/mL (>18 U/mL) by semi-quantitative interpretation using a HIT II-Ab ELISA kit (IBL international GmbH, Hamburg, Germany). No other antibodies with glycoprotein (GP) IIb/IIa, GP Ia/IIa, GP Ib/IX, or HLA were found by ELISA.
Discussion
HIT is a well-known severe adverse phenomenon induced by heparin. HIT involves platelet activation and thrombosis, which are paradoxically related to bleeding in the ICU (5). A case-control study on predominately cardiovascular surgery ICU patients found that a higher incidence of bleeding complications occurred in HIT patients (n=17 of 20; 85%) than in the control group (n=7 of 20; 35%) (6).
The incidence of HIT in surgery is reported to be higher than that of internal medicine, approximately 1–5% in patients given UFH (7). In this case, the patient was administered UFH, but at a very low dose, only give the pipe a flush before hemodialysis. The platelet count was assessed the day after the administration of UFH and was found to have decreased. Subsequently, the platelet count slightly increased after platelet transfusion; however, it decreased on the third day after a subsequent dose of UFH. The patient was exposed to heparin within 30 days, and the timing from inclusion to a drop in platelet count was ≤1. His platelet count decreased by >50% and the minimum platelet count was ≥20 G/L. The patient was taking the antibiotic Cefminox. In this case, the 4T score was 5, which indicates a high probability of HIT using the 4Ts scoring system. The postoperative immunological examination of anti-PF4/heparin IgG was positive (OD =1.205), and the concentration level was 19.957 U/mL (>18 U/mL), indicating the presence of antibodies against HIT. We note that over-treatment and inaccurate diagnosis should be avoided in order to prevent an exacerbation of symptoms. But in this case, heparin preparations were not be canceled after the patient was diagnosed with a high probability of HIT, intracerebral hemorrhage occurred while the patient was using heparin.
In conclusion, prognosis is good in cases where HIT is detected early. When the diagnosis is inconclusive, and the 4T score is above 4, all heparin preparations should be withdrawn immediately. Patients diagnosed with HIT generally do not need prophylactic platelet transfusion, which can lead to secondary activated platelet aggregation, increasing the risk of thrombosis.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2019.06.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Greinacher A. Heparin-induced thrombocytopenia: frequency and pathogenesis. Pathophysiol Haemost Thromb 2006;35:37-45. [Crossref] [PubMed]
- Watson H, Davidson S, Keeling D, et al. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. Br J Haematol 2012;159:528-40.
- Krzych ŁJ, Nowacka E, Knapik P. Heparin-induced thrombocytopenia. Anaesthesiol Intensive Ther 2015;47:63-76. [Crossref] [PubMed]
- Lo GK, Juhl D, Warkentin TE, et al. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost 2006;4:759-65. [Crossref] [PubMed]
- Warkentin TE, Greinacher A. Management of heparin-induced thrombocytopenia. Curr Opin Hematol 2016;23:462-70. [Crossref] [PubMed]
- Wester JP, Haas FJ, Biesma DH, et al. Thrombosis and hemorrhage in heparin-induced thrombocytopenia in seriously ill patients. Intensive Care Med 2004;30:1927-34. [Crossref] [PubMed]
- Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia: recognition, treatment, and prevention: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126:311S-337S. [Crossref] [PubMed]
Cite this article as: Liu J, Wang J, Deng J, Xu X, Shao Y, Chen Y, Ding H, Chen D, Ye X, Xia W. A case of unfractionated heparin-induced thrombocytopenia during the treatment of a gastric stromal tumor. Ann Blood 2019;4:20.