Comparison of three immunoassay systems for screening of HIV infection in blood donation in China
Introduction
HIV continues to be one of the major threats to public health worldwide as well as one of the major transfusion-transmitted infections. WHO reported there were approximately 36.7 million people suffering from HIV at the end of 2016, among which, 1.8 million were newly infected (1). Chinese Center for Disease Control and Prevention reported approximately 64 thousand people living with HIV infection as of May 2016, and a rapid increase of sexual transmission of HIV which signals a potential risk of the virus spreading to the general population (2). In China, HIV prevalence in the first-time blood donors was estimated to be 66/100,000 (95% CI: 59–74) and HIV incidence among repeat donors was estimated to be 9/100,000 (95% CI: 7–12) person-years (3).
Serological screening for HIV antibody/antigen was performed routinely using two EIAs in parallel in China for decades as required by government policy (4). If either of the two EIAs demonstrated reactive result, the blood donation will be discard and the blood donor will be deferred permanently. In 2015, the new Guidelines for Blood Banks Operation Procedures issued by the National Health and Family Planning Commission of the People’s Republic of China permitted the use of one EIA (single-EIA strategy) or one CLIA (single-CLIA strategy) for HIV antibody/antigen testing in parallel with nucleic acid testing (NAT). Chemiluminescent immunoassay (CLIA) has been extensively used for clinical diagnosis in China for years (5). Compared to EIAs, CLIA offers several advantages including (I) reduced testing time to less than one hour due to shorter incubation and reaction times by fully automated analyzers and (II) being suitable for use in random access mode, with ability to process specimens one at a time as per need, rather than in batches.
Given that there is no reported data comparing the recently allowed single-EIA, single-CLIA strategies to the two-different-EIAs strategy, we conducted testing using the Chemiluminescence microparticle immunoassay (CMIA). CMIA is a CLIA assay that is being used for door screening in the majority of blood centers in the developed world. We compared the performance of CMIA with the single-EIA and two-different-EIA testing results using as a gold standard the confirmatory results from the Western Blot test.
Methods
This study has obtained ethics approval from the ethics committee of the Institute of Blood Transfusion, Chinese Academy of Medical Sciences and the number of the approval is 201620.
Specimens
Blood donor samples were obtained from blood centers from four geographically diverse Chinese regions (Figure 1). Plasma samples from a total of 2,138 donors were collected. All samples had have gone through routinely screening for HBsAg, anti-HCV, anti-syphilis and HIV-1/2 Ab/Ag by two different licensed EIAs at the blood centers. Six different EIA kits used by the four blood centers are listed in Table 1.
Table 1
| Blood center | Kits for the detection of HIV Ab/Ag | Number of the samples |
|---|---|---|
| Chongqing | EIA kit for the detection of Anti-HIV (1+2), Shanghai Kehua Bio-engineering Co., Ltd. (Shanghai) | 2,033 |
| Diagnostic Kit for Antibodies to Human Immunodeficiency Virus (ELISA), KINGHAWK PHARMACEUTICAL (Beijing) | ||
| Mianyang | Diagnostic Kit for Antibodies to Human Immunodeficiency Virus (ELISA), ZHUHAI LIVZON DIAGNOSTIC INC. (Zhuhai) | 9 |
| GENSCREEN ULTRA HIV Ag-Ab, BIO-RAD (France) | ||
| Urumqi | Antibody to HIV 1+2 ELISA, Beijing Wantai Biological Pharmacy Enterprise CO., LTD. (Beijing) | 20 |
| Diagnostic Kit for Antibodies to Human Immunodeficiency Virus (ELISA), Biomerieux (Shanghai) | ||
| Guangxi | EIA kit for the detection of Anti-HIV (1+2), Shanghai Kehua Bio-engineering Co., Ltd. (Shanghai) | 76 |
| GENSCREEN ULTRA HIV Ag-Ab, BIO-RAD (France) |
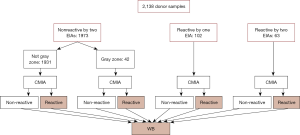
As showed in Table 2, among the 2,138 samples, 63 were tested reactive by both of the two EIAs, 102 samples were reactive by only one EIA and 1,973 samples were nonreactive by both of the two EIAs including 42 samples with gray zone results by one EIA and nonreactive by another.
Table 2
| Routine HIV Ab/Ag screening results by two EIAs | Number of the samples |
|---|---|
| Reactive by two EIAs | 63 |
| Reactive by one EIA | 102 |
| Nonreactive by two EIAs | 1,931 |
| Gray zone* by one EIA | 42 |
| Total | 2,138 |
#, all samples were freshly frozen after collection before shipped to IBT and restored at −80 °C; *Gray zone: samples tested with border line results (0.5< S/CO <1) by EIA.
HIV Ag/Ab screening by CMIA
The collected plasma samples were blinded to EIA results and assigned unique new sample identification numbers before tested testing with Abbott® HIV Ag/Ab Combo test (Abbott Diagnostics, Lake Forest, IL, USA) on the automated chemiluminescent microparticle immunoassay analyzer ARCHITECT i2000 system (CMIA, Abbott Diagnostic, Lake Forest, IL, USA). HIV Ag/Ab Combo assay is based on a two-step sandwich chemiluminescent microparticle immunoassay for the testing of anti-HIV antibody and HIV p24 antigen in plasma samples as previously described (5). All procedures were conducted in accordance with manufacturer’s instruction. Testing results were expressed as a signal to cut-off (S/CO) and S/CO >1.0 is considered reactive. The sensitivity and the specificity of this assay are 100% and 99.89% (95% CI: 99.68–99.98) respectively according to manufacture’s instruction.
Confirmatory testing by Western blot
Samples tested reactive (or gray zone) by either of the EIAs or CMIA were tested further by Western blot (WB, HIV Blot 2.0, MP Diagnostics, Singapore), an assay widely used for anti-HIV antibody confirmatory testing. Confirmed HIV infection status is defined by a positive result in WB testing. No further testing was performed on the samples tested nonreactive by both EIAs and CMIA. The algorithm of the screening and confirmatory testing is presented in Figure 2.
Statistical analysis
Statistical analysis was conducted using statistical software SAS 9.13 (SAS Institute, Cary, USA). Agreement between different strategies was interpreted as Kappa value, and the Kappa value more than 0.75 was considered as substantial agreement.
Results
Of the total 2,138 samples tested for HIV Ag/Ab by CMIA on the ARCHITECT i2000 system, 82 samples were reactive and 2,056 were nonreactive. All EIA reactive or gray-zone samples and all the CMIA reactive samples (219 in total) were tested further by WB. As shown in Table 3, 55 (55/58, 94.83%) of the 58 samples reactive by both EIAs and CMIA were confirmed by WB. Five samples (5/63, 7.94%) reactive by both of the two EIAs were nonreactive by CMIA, of which 1 (1/5, 20%) was confirmed reactive by WB. For the 102 samples tested reactive by only one of the two EIAs, nine (9/102, 8.82%) were reactive by CMIA and two of these (2/9, 22.22%) were confirmed positive and one (1/9, 11.11%) was indeterminate by WB. Of the 93 samples reactive by only one EIA and nonreactive by CMIA, none was confirmed positive by WB. Of the 42 samples with gray zone result by one EIA, three (3/42, 7.14%) were tested reactive by CMIA and 1 (1/3, 33.33%) were confirmed positive by WB. Of the 39 samples reactive with gray zone result by one EIA and nonreactive by CMIA, none was confirmed positive by WB.
Table 3
| Routine HIV Ab/Ag screening results by two EIAs | Results of CMIA | Results of WB, n (%) | Total | ||
|---|---|---|---|---|---|
| P | N | IND | |||
| Two EIAs reactive | Reactive | 55 (94.83) | 1 (1.72) | 2 (3.45) | 58 |
| Two EIAs reactive | Nonreactive | 1 (20.00) | 4 (80.00) | 0 (0.00) | 5 |
| One single EIA reactive | Reactive | 2 (22.22) | 6 (66.67) | 1 (11.11) | 9 |
| One single EIA reactive | Nonreactive | 0 (0.00) | 90 (96.77) | 3 (3.23) | 93 |
| Two EIAs nonreactive | Reactive | 4 (33.33) | 5 (41.67) | 3 (25.00) | 12 |
| Gray zone* by one EIA | Reactive | 1 (33.33) | 2 (66.67) | 0 (0.00) | 3 |
| Gray zone* by one EIA | Nonreactive | 0 (0.00) | 39 (100.00) | 0 (0.00) | 39 |
| Total | 63 (28.77) | 147 (67.12) | 9 (4.11) | 219 | |
*Gray zone: samples with border line results (0.5< S/CO <1). WB, Western blot; P, positive; N, negative; IND, indeterminate.
As showed in Table 4, 2,102 samples including 60 HIV-infected samples and 2,042 HIV-uninfected samples were tested by both Kehua ELISA and CMIA. Kehua ELISA correctly identified 54 samples as HIV-infected with a positive coincidence rate of 90.00% (95% CI: 79.49–96.24%) and 2,021 samples as uninfected with a negative coincidence rate of 98.97% (95% CI: 98.43–99.36%). A total of 2,027 samples including 47 HIV-infected samples and 1,980 HIV-uninfected samples were tested by both Kinghawk ELISA and CMIA. Kinghawk ELISA and CMIA correctly identified 43 (43/47, 91.49%) and 46 (46/47, 97.87%) HIV infected cases, respectively. Of the 17 HIV uninfected cases tested by both Wantai ELISA and CMIA, 15 (15/17, 88.24%) were correctly identified by CMIA while only one (1/17, 5.88%) was correctly identified by Wantai ELISA. Of the 7 HIV uninfected samples tested by both LIZON ELISA and CMIA, the CMIA correctly identified all (7/7, 100%) the seven samples while LIZON ELISA identified only 1 (1/7, 14.29%) samples correctly. The overall positive coincidence rate and the negative coincidence rate of HIV Ag/Ab CMIA were 98.41% (95% CI: 91.47–99.59%) and 99.32% (95% CI: 98.87–99.63%), respectively. The agreement between HIV infection status and different screening assays including CMIA and six different EIAs were compared, and the best consistency (99.38%) was found between CMIA and HIV infection status with a kappa value of 0.90.
Table 4
| CMIA and different EIAs | HIV infected cases | Positive coincidence rate (%) | 95% CI | HIV uninfected cases | Negative coincidence rate (%) | 95% CI | Consistency (%) | Kappa |
|---|---|---|---|---|---|---|---|---|
| Group 1a | 60 | 2,042 | ||||||
| CMIA correctly identified | 59 | 98.33 | 91.06–99.96 | 2,030 | 99.41 | 98.98–99.70 | 99.38 | 0.90 |
| Kehua correctly identified | 54 | 90.00 | 79.49–96.24 | 2,021 | 98.97 | 98.43–99.36 | 98.62 | 0.79 |
| Group 2b | 47 | 1,980 | ||||||
| CMIA correctly identified | 46 | 97.87 | 88.71–99.95 | 1,974 | 99.7 | 99.34–99.89 | 99.65 | 0.93 |
| Kinghawk correctly identified | 43 | 91.49 | 79.62–97.63 | 1,922 | 97.07 | 96.23–97.77 | 96.71 | 0.56 |
| Group 3c | 2 | 17 | ||||||
| CMIA correctly identified | 2 | 100 | 15.81–100.00* | 15 | 88.24 | 63.56–98.54 | 89.47 | 0.61 |
| Wantai correctly identified | 2 | 100 | 15.81–100.00* | 1 | 5.88 | 1.49–28.69 | 15.79 | 0.01 |
| Group 4d | 14 | 69 | ||||||
| CMIA correctly identified | 14 | 100 | 76.84–100.00* | 63 | 91.3 | 82.03–96.74 | 92.77 | 0.78 |
| Bio-Rad correctly identified | 12 | 85.71 | 57.19–98.22 | 65 | 94.2 | 85.82–98.40 | 92.77 | 0.76 |
| Group 5e | 1 | 7 | ||||||
| CMIA correctly identified | 1 | 100 | 2.50–100.00* | 7 | 100 | 59.04–100.00* | 100 | 1.00 |
| LIZON correctly identified | 1 | 100 | 2.50–100.00* | 1 | 14.29 | 3.61–57.87 | 25.00 | 0.04 |
| Group 6f | 3 | 16 | ||||||
| CMIA correctly identified | 2 | 66.67 | 9.43–99.16 | 15 | 93.75 | 69.77–99.84 | 89.47 | 0.60 |
| Biomerieux correctly identified | 2 | 66.67 | 9.43–99.16 | 16 | 100 | 79.41–100.00* | 94.44 | 0.76 |
| Group 7g | 63 | 2,066 | ||||||
| CMIA total correctly identified | 62 | 98.41 | 91.47–99.59 | 2,052 | 99.32 | 98.87–99.63 | 99.29 | 0.89 |
a, samples with definite HIV infection status tested by both CMIA and Kehua ELISA; b, samples with definite HIV infection status tested by both CMIA and Kinghawk ELISA; c, samples with definite HIV infection status tested by both CMIA and Wantai ELISA; d, samples with definite HIV infection status tested by both CMIA and Bio-Rad ELISA; e, samples with definite HIV infection status tested by both CMIA and LIZON ELISA; f, samples with definite HIV infection status tested by both CMIA and Biomerieux ELISA; g, all the samples with definite HIV infection status tested by CMIA; *, one-sided, 97.5% confidence interval.
Discussion
Before 2016, all Chinese blood donors underwent HIV screening by two-different-EIAs. Recently, CLIA has been authorized by the Chinese government for the detection of HIV infection in blood donors. CLIA offers several advantages over traditional EIA screening, including less labor-intensity, higher throughput and faster turn-around time (6). For instance, the Abbott CMIA (one type of CLIA) platform used in this study has a throughput of 200 tests per hour with a turn-around of 28 minutes (7). In contrast, EIA needs a long incubation time which results in delays in results reporting time and might increase the overall testing cost for some small blood centers. Numerous studies have reported that the HIV Combo CLIA has excellent sensitivity and specificity. A study of 10,995 specimens from different US studies funded by the Centers for Disease Control and Prevention reported that the sensitivity of CLIA for the detection of HIV Ag/Ab was 99.94% (95% CI: 99.79–99.99) and the specificity was 98.78% (95% CI: 98.51–99.01), and 48 (83%) of 58 HIV RNA positive but EIA nonreactive specimens were detected by CLIA (8). Clinical performance of HIV Ag/Ab combination CMIA was investigated in China by testing of 88,000 clinical specimens and reported the sensitivity as 100% and specificity as 99.93% (95% CI: 99.73%–99.99%) (9). A study of 35,420 samples from a Chinese hospital compared the performance of EIA and CLIA. Eight (80%) of the 10 samples tested CLIA reactive but EIA nonreactive were confirmed positive by WB while no WB positive samples were missed by CLIA (10). Ouyang et al. (11) reported that among the 59 acute HIV infected specimens (37 were collected from a follow-up study of men who have sex with men in China, and the others were from seroconservation panels of BBI and NABI and NIBSC companies), the sensitivity of CLIA (96.61%) was significantly higher than that of EIA (83.93%), and among the 703 specimens from clinical patients, the specificity of CLIA and EIA was 100% and 99.71%, respectively.
In the present study, we evaluated the performance of HIV 1/2 antibody/antigen CMIA in volunteer blood donors from four Chinese blood centers using WB as the confirmatory test for HIV infection status. We found that the HIV 1/2 antibody/antigen CMIA has a positive coincidence rate of 98.41% (95% CI: 91.47–99.59%) and a negative coincidence rate of 99.32% (95% CI: 98.87–99.63) with WB results, both higher than or equal to that of EIAs except for Bio-rad EIA and BioMerieux EIA. Bio-rad EIA showed a lower positive coincidence but a higher negative coincidence than CMIA while BioMerieux EIA showed the same positive coincidence with CMIA but a slightly higher negative coincidence rate. However, given that the relatively small sample size in these two groups (69 samples in group 4 and 19 samples in group 6), further study need to be conducted to gain more convincing results. In addition, good agreement (Kappa value higher than 0.75) with HIV infection status was found by CMIA (0.90), Kehua ELISA (0.79), BioMerieux ELISA (0.76) and Bio-Rad ELISA (0.76), among which CMIA has the best agreement with confirmed HIV infection status.
To compare the three strategies of two-different-EIAs, single-EIA and single-CMIA, the confirmed infection status of the samples with discrepant results by different strategies were further analyzed. The two-different-EIAs strategy demonstrated a very high false positive rate (all 93 samples disqualified by only one of the two EIAs were nonreactive by CMIA and negative by WB). Both single-CMIA strategy and single-EIA strategy demonstrated a lower false positive rate than the two-different-EIAs strategy. Of the 219 samples with discrepant results by EIAs and CMIA, 4 samples were reactive by single-CMIA but were missed by two-different-EIAs strategy and only one sample was missed by single-CMIA while reactive by the two-different-EIAs strategy. Therefore, the single-CMIA strategy demonstrated a lower false negative rate than two-different-EIAs and single-EIA strategy. To improve the sensitivity of two-different-EIAs, many blood centers in China set gray zone as S/CO between 0.5 and 1.0 and disqualify all donations with a gray zone or reactive result by either of the two EIAs. Only one of the 219 samples tested as gray zone was confirmed positive and this sample was detected as reactive by the single-CMIA strategy. Our results indicate that even though the use of a gray zone does increase the sensitivity of HIV screening with EIAs, it also significantly increased the rate of disqualifying donations based on false negative results.
There are several limitations relating to interpretation of results from this study. Firstly, only samples with screening reactive or gray zone EIA results were utilized in this study. Thus, our results cannot be used to calculate the true sensitivity and specificity of EIAs and CMIAs. Secondly, since different EIA kits were used by the four blood centers, the numbers of samples tested by a certain EIA kit are relatively small. This can affect the evaluation of the performance of different EIAs. The low agreement between HIV infection status with LIZON EIA and Wantai EIA in this study may be related to the small numbers of samples screened by these two assays included in this study. Thirdly, we didn’t include the NAT results of these samples because the aim of this study is to compare the performance of three serological screening strategies. Moreover, the study was performed prior to implementation of NAT in China and hence NAT data is not available on all the samples included in this evaluation. The data on subset of samples where NAT data was available identified two samples that were negative by NAT, however, positive by CMIA and confirmed by the WB (data not shown). Whereas, the study also identified two samples that were NAT positive but negative by CMIA. These may be due to below the antigen detection limit of the CMIA with low viral load (data not shown). In addition, there were 48 samples that were NAT positive and all were detected by the CMIA (data not shown). We believe that improving the effectiveness of both serological and NAT screening is important to maximize blood safety.
This is the first study to compare the performance of the recently approved single-EIA, single-CLIA donor screening strategies with the currently used two-different-EIAs strategy for HIV blood screening in China. Our results suggest that single-CLIA performed better than both EIA strategies according to the agreements of results from these assays with the confirmed HIV infection status. The implementation of the single-CMIA strategy may serve to further reduce the risk of transfusion transmitted HIV infection and avoid the need for multiple assays, while decrease unnecessary waste of blood caused by false positive results.
Acknowledgments
The authors thank the blood centers for providing the samples and the data.
Funding: This work was supported by Abbott Diagnostics and the CAMS Innovation Fund for Medical Sciences (CIFMS, No. 2016-I2M-1-018).
Footnote
Conflicts of Interest: The authors Sushil Devare, Jian-Fang Liu, Xiao-Ting Lv, and Peng Yin are employees of Abbott Diagnostics. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual informed consent was waived. This study has obtained ethics approval from the ethics committee of the Institute of Blood Transfusion, Chinese Academy of Medical Sciences and the number of the approval is 201620.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. HIV/AIDS. Available online: http://www.who.int/mediacentre/factsheets/fs360/en/, accessed November 2016.
- China CDC. The HIV/AIDS epidemic in China. Chinese Journal of AIDS & STD 2016;9:675.
- Wang J, Liu J, Yao F, et al. Prevalence, incidence, and residual risks for transfusion-transmitted human immunodeficiency virus Types 1 and 2 infection among Chinese blood donors. Transfusion 2013;53:1240-9. [Crossref] [PubMed]
- Zeng P, Liu J, Wang J, et al. Parallel enzyme-linked immunosorbent assay screening for human immunodeficiency virus among blood donors in five Chinese blood centres: a retrospective analysis. Transfus Med 2015;25:259-64. [Crossref] [PubMed]
- Kaye D. FDA Approves First Diagnostic Assay to Detect Both HIV Antigen and Antibodies. Clinical Infectious Diseases 2010;
- Manlutac AL, Giesick JS, McVay PA. Identification of early HIV infections using the fourth generation Abbott ARCHITECT HIV Ag/Ab Combo chemiluminescent microparticle immunoassay (CIA) in San Diego County. J Clin Virol 2013;58:e44-7. [Crossref] [PubMed]
- Eshleman SH, Khaki L, Laeyendecker O, et al. Detection of individuals with acute HIV-1 infection using the ARCHITECT HIV Ag/Ab Combo assay. J Acquir Immune Defic Syndr 2009;52:121-4. [Crossref] [PubMed]
- Chavez P, Wesolowski L, Patel P, et al. Evaluation of the performance of the Abbott ARCHITECT HIV Ag/Ab Combo Assay. J Clin Virol 2011;52:S51-5. [Crossref] [PubMed]
- Cui C, Liu P, Feng Z, et al. Evaluation of the clinical effectiveness of HIV antigen/antibody screening using a chemiluminescence microparticle immunoassay. J Virol Methods 2015;214:33-6. [Crossref] [PubMed]
- Duan M, Lan L. Comparison of CLIA and ELISA in HIV screening before surgery. Modern Instruments 2016;22:78-80.
- Ouyang J, Han X, Ji Y, et al. A comparative study on the clinical performance of three fourth generation HIV diagnostic reagents. Chinese Journal of Laboratory Medicine 2013;36:903-7.
Cite this article as: Wang MY, Devare S, Liu JF, Lv XT, Yin P, Guo N, Fu P, Wu BT, Yin YH, Ke L, Li X, Shan H, Liu Y. Comparison of three immunoassay systems for screening of HIV infection in blood donation in China. Ann Blood 2019;4:13.