
Anticoagulation therapy in Italy
Introduction
Venous and arterial thromboses are the leading cause of death and disability in high-income countries, and their incidence is also dramatically increasing in middle- and low-income countries (1). The burden of thromboembolic diseases imposes primary prevention as the most important community goal, through lifestyle interventions on a number of modifiable risk factors such as smoking, hypertension, abdominal obesity, physical inactivity and inadequate dietary habits. Despite the positive outcome associated with these measures, many people still develop clinical manifestations of thromboembolism, so that a pharmacological approach to secondary prevention and treatment is necessary to reduce morbidity and mortality. Since blood hypercoagulability plays a pivotal role in thrombogenesis, it is reasonable that anticoagulant agents should be regarded as an essential therapeutic tool in the management of these patients (2-7).
Heparins [unfractionated heparin (UFH) initially, and low-molecular weight heparins (LMWHs) later] and vitamin K antagonists (VKAs, warfarin, phenprocoumon, acenocoumarol) have been used for decades for treatment and prevention of thromboembolism (8). Over the past 15 years, however, the interest in anticoagulants has grown dramatically, as shown by the increasing number of drugs introduced in both preclinical and clinical development as well as by the vast array of anticoagulants currently licensed (see Table 1). In particular, investigators focused their research on the direct oral anticoagulants (DOACs), which selectively target specific steps of the coagulation cascade (9-14). The currently licensed DOACs include dabigatran, which selectively inhibits thrombin, and apixaban, edoxaban and rivaroxaban which selectively inhibit activated factor X (9,14). A large quantity of clinical experimental studies and real-world data have documented the clinical benefit and the high safety profile of DOACs in the thromboembolic setting (4). The main purpose of the current article is to summarize the contemporary organization of anticoagulation therapy in Italy, and to discuss both positive aspects and potential criticisms.
Table 1
| Parenteral |
| Heparins (UFH, LMWH) |
| Fondaparinux |
| Thrombin inhibitors (bivalirudin, lepirudin, argatroban) |
| Oral |
| VKAs |
| Thrombin inhibitors (dabigatran) |
| Factor Xa inhibitors (rivaroxaban, apixaban, edoxaban) |
UFH, unfractionated heparin; LMWH, low molecular weight heparin; VKAs, vitamin K antagonists.
VKA anticoagulation therapy in Italy
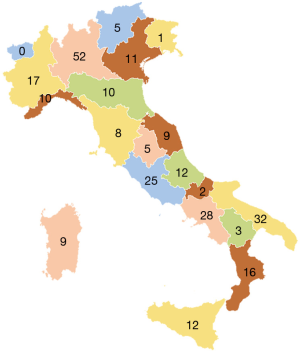
Like other western countries, thrombotic disease in Italy affects millions of people, being thus a significant health problem of great social relevance. An efficient instrumental and laboratory diagnosis of thrombotic clinical events, together with the clinical and laboratory surveillance of antithrombotic therapies is therefore a necessary requirement for our country. In Italy, anticoagulation treatment is practiced by physicians, mostly specialists in hematology, laboratory or internal medicine, operating in public or accredited hospital centers belonging in the majority of cases to Laboratory, Transfusion, Internal Medicine or Hematology Divisions. Starting from the previously mentioned considerations, some thrombosis centers decided to set up the Italian Federation of Centers for Diagnosis of Thrombosis and Surveillance of Antithrombotic Therapies (FCSA) around 40 years ago. There are 267 centers currently affiliated to FCSA (data updated to December 2018) and these are present in all Italian regions with the exception of Val D’Aosta region (see Figure 1). In most cases, these structures are configured as treatment centers for anticoagulant therapies, including prevention, diagnosis and treatment of thrombosis diseases and the management of treatment-related hemorrhagic complications. The specialized clinicians working in Italian FCSA centers collaborate closely with a number health professionals, including nurses, biologists, laboratory technicians, for a global number of over 2,000 operators, who follow approximately 300,000 patients treated with VKAs.
FCSA (website: www.fcsa.it) was founded in 1989 to assist patients with antithrombotic therapy and respond to their health needs. With this aim, since its foundation, FCSA promotes educational and cultural activities, organizes training courses for doctors, biologists, technicians, and nurses, and produces manuals for easy consultation for operators. In addition, for many years FCSA has activated a process of professional accreditation to improve skills and expertise of health professionals operating at the specialized centers. FCSA is also active in the field of clinical and laboratory research in order to promote the improvement of knowledge mainly in the management of patients in anticoagulant therapy and, at the same time, the professional growth of all health operators. This scientific activity has led to the realization of a number of studies published in international scientific journals of high impact in the last years (15-20).
FCSA collaborates with local authorities (municipals and Regions), as well as national (National Institute of Health, State-Regions Conference, Ministry of Health) and regulatory (Italian Drug Agency, AIFA) authorities, to optimize diagnostic-therapeutic pathways in patients receiving antithrombotic therapies and to increase epidemiological, pharmacologic and pharmacoeconomic knowledge.
FCSA has been in these years the guide for affiliated thrombosis centers promoting the following activities:
- Support the centers from a scientific and organizational point of view;
- Make physicians and care givers aware of the health and social importance of an effective surveillance of antithrombotic therapy;
- Create guidelines and recommendations on the topic of antithrombotic therapy;
- Promote the standardization of laboratory methods and the comparability of the results produced in different centers through the implementation of specific external quality assessment programs;
- Contribute to the continuous updating of the staff of the centers;
- Promote conferences and workshops on topics of interest;
- Promote and perform multicenter clinical studies to implement our knowledge on critical issues.
In addition, FCSA collaborates closely with the Italian Federation of Anticoagulated Patients (FEDER-AIPA), a voluntary association founded in 1995 and which currently includes 60 sections in the various Italian regions. The mission of FEDER-AIPA is to improve the quality of life of Italian anticoagulated patients through collaboration with professionals of thrombosis centers and scientific associations, mainly FCSA, and a continuous dialogue with the representatives of the administrative and health structures to promote the patients’ needs.
The main objective of FCSA for the next few years is to implement the activity of the affiliated thrombosis centers, transforming them from structures mainly dedicated to the surveillance of anticoagulant therapy to centers that care globally for the patients with thrombosis, from diagnosis to the instrumental and laboratory diagnosis to the antithrombotic treatment and follow-up.
DOAC anticoagulation therapy in Italy
In the last 5 years, like other western countries, in Italy there has been an exponential increase in the prescription of DOACs and it has been estimated that more than 100,000 Italian patients are currently taking these new oral drugs. DOACs are distributed free in Italy through the online compilation of a therapeutic plan regulated by AIFA (https://servizionline.aifa.gov.it/). Only specialists in hematology, pulmonology, cardiology, neurology, vascular surgery can be qualified to compile these therapeutic plans. This online service acts also as a register for patients under VKAs and for monitory drugs-related adverse events with all anticoagulants.
According to the European Heart Rhythm Association (EHRA) guidelines (21), patients taking DOACs are to be laboratory and clinically monitored every 3–6 months. In Italy, this follow-up is usually performed at FCSA thrombosis centers. FCSA collaborates also with these centers for the management of such patients. In addition, this association promotes training courses for health professionals of thrombosis centers and clinical research to improve our knowledge on the post-marketing assessment of safety and efficacy of these recently commercialized anticoagulant agents.
As a supplement to this process, FEDER-AIPA gives its support to centers and patients, being aware that also individuals receiving DOACs need support from professionals and volunteers to better understand their underlying pathologic condition and all benefits and possible risks of the new oral drugs that they are taking.
An open issue remains the monitoring of DOAC activity in emergency conditions, such as bleeding, trauma, urgent surgery and renal failure (22-26). Although in these critical situations it is usually recommended that DOAC measurement be undertaken (12), not all Italian hospitals are equipped for this eventuality. FCSA, in collaboration with the Italian Society of Clinical Biochemistry and Clinical Molecular Biology (SIBioC), is working with Italian regulatory agencies to allow such activity at least in those hospitals where a thrombosis center exists, also considering the increasing number of patients anticoagulated with DOACs.
Discussion
The current organization of anticoagulation therapy in Italy involves a number of professionals operating at the FCSA thrombosis centers with the aim of following closely those patients taking VKAs or DOACs. This organizational model has been developed during the past years, implementing an increasing number of centers all over Italy in order to reach a global network for the clinical assistance of all Italian anticoagulated patients.
The progress of the standard of care for such patients over the last 20 years has resulted in better patient management, with an improvement of health-related quality of life and patients’ clinical outcomes. For instance, the mean Time in Therapeutic Range (TTR) of VKA-treated patients followed by FCSA thrombosis centers in Italy in 2018 was nearly 70% (unpublished data, presented orally at the last FCSA congress held in Bologna, Italy, 05–07 October 2018), significantly higher than that reported in other countries with different models of patients’ management or even in the clinical trials that evaluated DOACs (27,28). The evidence of the high quality of care reached by FCSA thrombosis centers emerged also from a recent study conducted in Italy by Palareti and colleagues (29). In this prospective multicenter trial on VKA-treated patients, the authors compared the currently observed clinical results with those recorded 20 years before from the FCSA-START-Register (30). A marked reduction of thrombotic complications and a lower mortality rate was observed in the more recent cohort of patients (29), documenting that important changes in the management of anticoagulated patients have occurred during the period between the two studies.
Unfortunately, there is no rose without thorns. The other side of this model of care is the higher economic impact for health organizations and the increased burden for physicians operating at the FCSA thrombosis centers, in a socioeconomic period characterized by ever lower economic resources and a severe shortage of health personnel. Thus, the maintenance of such a model represents a major challenge for our country.
With this background, in order to meet the needs of an increasing number of orally anticoagulated patients, FCSA has recently developed a collaboration with the Italian Society of General Medicine (SIMG). The resulting consensus document, available online: http://www.fcsa.it/Content/DocumentiChiSiamo/DOCUMENTO%20FINALE%20CONSENSUS%20SIMG%20FCSA%20febbraio%202018.pdf, includes the management of anticoagulated patients by general practitioners (who are responsible for the health status of patients), following an adequate training and in close link with specialists of thrombosis center, particularly during the follow-up period.
Conclusions
Anticoagulation therapy in Italy has evolved in the last two decades, greatly improving the way in which patients with thrombosis are managed. The socioeconomic burden of such an organizational model is, however, high and requires continuous resources directed towards the implementation of the staff of thrombosis centers to respond to the growing demand arising from the increasing number of patients now undergoing anticoagulation therapy. This increase in anticoagulation results both from the increasingly aging of the population, with a well-known association of increased thrombotic risk according to age, as well as the perceptions around increasing safety of DOACs driving an increase in anticoagulation of patients less suited to VKA therapy. The progressive involvement of adequately trained general practitioners working in close collaboration with FCSA centers could represent a solution to resolve this critical issue, overcoming the current imbalance between patients requiring anticoagulation treatment and the availability and experience of their caregivers.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Emmanuel J. Favaloro) for the series “Anticoagulant and antithrombotic therapy: globally applied according to local geographical selection criteria” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob. 2019.02.01). The series “Anticoagulant and antithrombotic therapy: globally applied according to local geographical selection criteria” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mannucci PM, Franchini M. Old and new anticoagulant drugs. Ann Med 2011;43:116-23. [Crossref] [PubMed]
- Eikelboom JW, Weitz JI. Update on antithrombotic therapy. New anticoagulants. Circulation 2010;121:1523-32. [Crossref] [PubMed]
- Hirsh J. Oral anticoagulant drugs. N Engl J Med 1991;324:1865-75. [Crossref] [PubMed]
- Lip GY, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest 2018;154:1121-201. [Crossref] [PubMed]
- Franchini M, Mannucci PM. A new era for anticoagulants. Eur J Intern Med 2009;20:562-8. [Crossref] [PubMed]
- Franchini M, Liumbruno GM, Bonfanti C, et al. The evolution of anticoagulant therapy. Blood Transfus 2016;14:175-84. [PubMed]
- Favaloro EJ. Anticoagulant therapy: present and future. Semin Thromb Hemost 2015;41:109-12. [Crossref] [PubMed]
- Baluwala I, Favaloro EJ, Pasalic L. Therapeutic monitoring of unfractionated heparin - trials and tribulations. Expert Rev Hematol 2017;10:595-605. [Crossref] [PubMed]
- Jackson LR 2nd, Becker RC. Novel oral anticoagulants: pharmacology, coagulation measures, and considerations for reversal. J Thromb Thrombolysis 2014;37:380-91. [Crossref] [PubMed]
- Lim HY, Nandurkar H, Ho P. Direct oral anticoagulants and the paradigm shift in the management of venous thromboembolism. Semin Thromb Hemost 2018;44:261-6. [Crossref] [PubMed]
- Iapichino GE, Bianchi P, Ranucci M, et al. Point-of-care coagulation tests monitoring of direct oral anticoagulants and their reversal therapy: state of the art. Semin Thromb Hemost 2017;43:423-32. [Crossref] [PubMed]
- Favaloro EJ, Pasalic L, Curnow J, et al. Laboratory monitoring or measurement of direct oral anticoagulants (DOACs): Advantages, limitations and future challenges. Curr Drug Metab 2017;18:598-608. [Crossref] [PubMed]
- Lippi G, Mattiuzzi C, Cervellin G, et al. Direct oral anticoagulants: analysis of worldwide use and popularity using Google Trends. Ann Transl Med 2017;5:322. [Crossref] [PubMed]
- Ntaios G, Papavasileiou V, Makaritsis K, et al. Real-world setting comparison of nonvitamin-K antagonist oral anticoagulants versus vitamin-K antagonists for stroke prevention in atrial fibrillation: a systematic review and meta-Analysis. Stroke 2017;48:2494-503. [Crossref] [PubMed]
- Poli D, Antonucci E, Pengo V, et al. Mechanical prosthetic heart valves: Quality of anticoagulation and thromboembolic risk. The observational multicenter PLECTRUM study. Int J Cardiol 2018;267:68-73. [Crossref] [PubMed]
- Tripodi A, Ageno W, Ciaccio M, et al. Position Paper on laboratory testing for patients on direct oral anticoagulants. A Consensus Document from the SISET, FCSA, SIBioC and SIPMeL. Blood Transfus 2018;16:462-70. [PubMed]
- Tosetto A, Manotti C, Marongiu F, et al. Center-Related Determinants of VKA Anticoagulation Quality: A Prospective, Multicenter Evaluation. PLoS One 2015;10:e0144314 [Crossref] [PubMed]
- Riva N, Ageno W, Poli D, et al. Safety of vitamin K antagonist treatment for splanchnic vein thrombosis: a multicenter cohort study. J Thromb Haemost 2015;13:1019-27. [Crossref] [PubMed]
- Poli D, Antonucci E, Dentali F, et al. Recurrence of ICH after resumption of anticoagulation with VK antagonists: CHIRONE study. Neurology 2014;82:1020-6. [Crossref] [PubMed]
- Cosmi B, Legnani C, Pengo V, et al. The influence of factor V Leiden and G20210A prothrombin mutation on the presence of residual vein obstruction after idiopathic deep-vein thrombosis of the lower limbs. Thromb Haemost 2013;109:510-6. [Crossref] [PubMed]
- Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association practical guide on the use of non-vitamin-K antagonist anticoagulants in patients with non-valvular atrial fibrillation: Executive summary. Eur Heart J 2017;38:2137-49. [PubMed]
- Lippi G, Favaloro EJ. Laboratory monitoring of direct oral anticoagulants (DOACs)-The perfect storm? Ann Transl Med 2017;5:6. [Crossref] [PubMed]
- Cervellin G, Benatti M, Bonfanti L, et al. Quality and safety issues of direct oral anticoagulants in the emergency department. Semin Thromb Hemost 2015;41:348-54. [Crossref] [PubMed]
- Favaloro EJ, Lippi G. Laboratory testing in the era of direct or non-vitamin K antagonist oral anticoagulants: a practical guide to measuring their activity and avoiding diagnostic errors. Semin Thromb Hemost 2015;41:208-27. [Crossref] [PubMed]
- Gosselin RC, Adcock DM, Bates SM, et al. International Council for Standardization in Haematology (ICSH) Recommendations for Laboratory Measurement of Direct Oral Anticoagulants. Thromb Haemost 2018;118:437-50. [Crossref] [PubMed]
- Lippi G, Favaloro EJ. Recent guidelines and recommendations for laboratory assessment of the direct oral anticoagulants (DOACs): is there consensus? Clin Chem Lab Med 2015;53:185-97. [Crossref] [PubMed]
- Kimmel SE, French B, Kasner SE, et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med 2013;369:2283-93. [Crossref] [PubMed]
- Senoo K, Lip GY. Comparative Efficacy and Safety of the Non-Vitamin K Antagonist Oral Anticoagulants for Patients with Nonvalvular Atrial Fibrillation. Semin Thromb Hemost 2015;41:146-53. [Crossref] [PubMed]
- Palareti G, Antonucci E, Migliaccio L, et al. Vitamin K antagonist therapy: changes in the treated populations and in management results in Italian anticoagulation clinics compared with those recorded 20 years ago. Intern Emerg Med 2017;12:1109-19. [Crossref] [PubMed]
- Palareti G, Leali N, Coccheri S, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on complications of oral anticoagulant therapy. Lancet 1996;348:423-8. [Crossref] [PubMed]
Cite this article as: Franchini M. Anticoagulation therapy in Italy. Ann Blood 2019;4:5.


