Oral anticoagulants around the world: an updated state-of-the art analysis
Introduction
Venous and arterial thromboses are leading causes of morbidity, mortality and disability worldwide (1). The prevention and management of thrombotic disorders is achieved through the use of anticoagulant and antithrombotic drugs (2), including heparins (both unfractionated or low molecular weight heparin), vitamin K antagonists (VKAs; namely warfarin), as well as more recently developed agents, currently defined as direct oral anticoagulants (DOACs) (3). This name originates from the direct mechanism of action, with these drugs being designed to selectively inhibit thrombin (or activated factor II; i.e., dabigatran) or activated factor X (FXa; i.e., rivaroxaban, apixaban and edoxaban). Beside the specific biological activity, these innovative agents differ in many other ways from heparins and VKAs. Unlike heparins, DOACs are orally administered, and thus represent a more comfortable and potentially more compliant means for long-term prevention and treatment of thrombosis. Unlike warfarin, they are characterized by a much more predictable pharmacokinetic response in vivo, which would make laboratory monitoring less important, albeit not always unnecessary, as compared to the more conventional VKAs (4,5). Reliable data also suggests that the overall antithrombotic potential of DOACs is globally comparable to that of heparins and VKAs, whilst the risk of major and minor bleeding seems substantially lower (6). Altogether, these findings suggest that DOACs will soon become the standard of care for prevention and management of nearly all major thrombotic diseases, thus including atrial fibrillation, ischemic heart and cerebrovascular diseases, peripheral arterial occlusive disease (PAOD) and venous thromboembolism, among others (7,8). Nevertheless, VKAs and heparins will likely still have a place in treatment and prevention of thrombosis in selected clinical situations for the foreseeable future.
Google Trends (Google Inc. Mountain View, CA, USA) is a freely available web resource aimed at summarizing the overall number of Google searches over time and across different geographical settings. Thus, the output of Google Trends analysis for a given search terms directly reflects the volume of Google searched for that same term (9). Increasing evidence suggests that analyses of Google Trends may be reliably used for estimating the worldwide or local popularity—in terms of Google searches—of many pathological conditions and drug usage, thus leading the way to promote the dissemination of the so-called digital epidemiology (10).
A previous study, published over a year ago, showed that the popularity of DOACs searched in Google had considerably increased during the past decade, especially in certain parts of the world (11). Therefore, this study was aimed at using Google Trends to provide an update analysis or ‘snapshot’ of the current worldwide popularity of the commercially available oral anticoagulant agents.
Methods
An electronic search was carried in Google Trends, simultaneously using the search terms “warfarin” AND “dabigatran” AND “rivaroxaban” AND “apixaban” AND “edoxaban”. No language or geographical restrictions were applied, but the electronic search was limited to the past 30 days from the current search date (i.e., between October 5, 2018 and November 5, 2018) to enable this ‘snapshot’ of current popularity. According to the Google Trends algorithm, data were cumulatively arranged according to the relative peak of Google searches, where a value of 100 reflects the highest peak, a value of 50 signifies that the search term had half popularity compared to the peak of searches, and a value of 0 implies that the term was only searched <1% fold than the number of peak searches. The study was carried out in accordance with the Declaration of Helsinki and under the terms of relevant local legislation.
Results
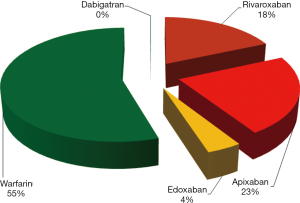
The results of the Google Trends search are shown in Figure 1. Overall, warfarin (55.0%) received the largest number of Google searches among the five oral anticoagulants throughout the study period. This was followed by apixaban (22.5%) and rivaroxaban (18.3%). A much lower number of Google searches was identified for edoxaban (4.1%), whilst the number of Google searches for dabigatran was minimal (0.1%) compared to those of the other drugs. The geographical distribution of the worldwide Google searches is shown in Figure 2 and basically reflects previously presented popularity data. Briefly, warfarin remains the predominantly searched oral anticoagulant agent around the world. Rivaroxaban was more popular in China and in some South-American countries, whilst apixaban was especially popular in some North-European and Latin American countries. In contrast, edoxaban and dabigatran did not currently appear to be popular drugs in any country worldwide. The geographical distribution of the worldwide Google searches for warfarin, apixaban, rivaroxaban and edoxaban is shown in Figure 3. Notably, the number of Google searches for dabigatran was so low that Google Trends failed to provide any geographical information. The 15-top areas with the highest Google searches for each oral anticoagulant drug (except dabigatran, for which insufficient searches were available) is shown in Table 1.
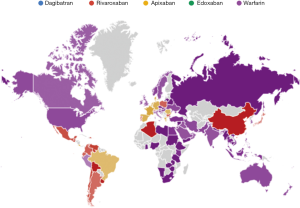
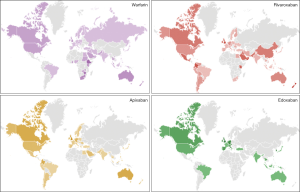
Table 1
| Rank | Warfarin | Apixaban | Rivaroxaban | Edoxaban | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Areas | Hits | Areas | Hits | Areas | Hits | Areas | Hits | ||||
| 1 | Ethiopia | 100 | United Kingdom | 100 | New Zealand | 100 | United Kingdom | 100 | |||
| 2 | Trinidad & Tobago | 96 | Lebanon | 62 | Cyprus | 97 | Germany | 82 | |||
| 3 | Zimbabwe | 93 | Ireland | 55 | Oman | 76 | Ireland | 82 | |||
| 4 | New Zealand | 61 | Australia | 55 | United Kingdom | 74 | Taiwan | 82 | |||
| 5 | South Africa | 61 | Canada | 54 | Uruguay | 72 | Italy | 80 | |||
| 6 | United Kingdom | 61 | Colombia | 52 | Singapore | 70 | South Korea | 73 | |||
| 7 | Qatar | 58 | Germany | 41 | Colombia | 67 | Austria | 58 | |||
| 8 | Bahrain | 58 | Singapore | 36 | Qatar | 63 | Spain | 58 | |||
| 9 | Australia | 58 | USA | 35 | Ireland | 58 | Switzerland | 53 | |||
| 10 | Cyprus | 51 | Hong Kong | 33 | Lebanon | 58 | Belgium | 47 | |||
| 11 | Ireland | 51 | Panama | 33 | Guatemala | 52 | Netherlands | 45 | |||
| 12 | Jordan | 51 | Netherlands | 32 | Saudi Arabia | 44 | Portugal | 37 | |||
| 13 | Malaysia | 45 | Saudi Arabia | 30 | Hong Kong | 43 | Canada | 35 | |||
| 14 | Singapore | 45 | United Arab Emirates | 29 | Mexico | 42 | USA | 24 | |||
| 15 | Czech Republic | 45 | Ecuador | 28 | St Helena | 38 | Thailand | 21 | |||
Discussion
The use of oral anticoagulant/antithrombotic therapy remains a mainstay in prevention and management of many arterial and venous thromboembolic diseases, and will likely do so for the foreseeable future. The differential use of these drugs has, however, undergone a remarkable revolution in the past decade, in particular after the new agents, conventionally called DOACs, have been gradually introduced into clinical practice. These have several advantages to the more conventional coumarin derivatives (i.e., the VKAs), which have been previously emphasized, and anticipate a brilliant marketing future for DOACs, with many more patients to be shifted to short- and long-term usage of these drugs.
However, this hypothetical scenario has yet to be fully played out, since the results of our update analysis on oral anticoagulant treatments reveal that the popularity of warfarin still remains particularly high around the world, and being cumulatively higher than that of all DOACs altogether. As shown in Figure 2 and Table 1, the popularity of Warfarin also remains globally widespread, with a presence in many countries worldwide. Of interest, VKAs represent the oldest, and thus the least expensive of oral anticoagulants available. Nevertheless, the popularity of VKAs such as warfarin is not just limited to low-income or emerging nations, but also includes the USA, Canada, Australia and Italy. The popularity of rivaroxaban and apixaban is also widespread around the world, with a discrete geographical overlap between these two DOACs. Unlike these highly popular drugs, dabigatran, the original DOAC to be released into the marketplace, now appears very modest, representing approximately ~0.1% of all worldwide Google searches for oral anticoagulant drugs. This result is not unexpected, since a previous similar analysis, carried out over a 1 year ago, also highlighted the incessantly decreasing trend of popularity of this drug during the previous 5 years.
Although a deep analysis of the causes which have led to the current scenario is outside the major aim of this study, some general hypotheses can be proposed. Warfarin has been for long past the standard of care in patients needing anticoagulant therapy; this situation was mostly due to the fact that no other reliable alternatives had been available (12). The commercialization of DOACs has then contributed to revolutionize the landscape of anticoagulant therapy, but the still broad popularity of warfarin is likely attributable to several factors. One possibility is a kind of resistance to changes that many physicians have to ‘experimenting’ with new drugs (13), as well as a lack of antidote and lack of clinical experience in reversing any new drug’s anticoagulant effects. This might also have some basis in the bad press received by the emerging DOACs when several adverse cases were presented in the popular press, due to over-marketing of these drugs to general practitioners as ‘wonder drugs’ that did not need monitoring (12). It is likely that DOACs were initially over-prescribed, and in some cases given to patients who may have had some contraindications (12). Another consideration is the still considerably lower cost of warfarin compared to DOACs (14,15). This probably explains why the popularity of warfarin remains very high in many low-income or emerging nations such as Ethiopia, Trinidad and Tobago, Zimbabwe, South Africa, Cyprus, Jordan and Malaysia. It is also worth mentioning here that the confidence that many clinicians have in DOACs remains frequently lower than with warfarin, a drug with which they have become comfortable over the many years of its use (16,17). This may be attributable to the fact that warfarin therapy necessitates continuous laboratory monitoring (i.e., by means of a narrow therapeutic range for prothrombin time/international normalized ratio; PT/INR) to assess its anticoagulant effect and thus preventing insufficient dose (and the subsequent enhanced risk of thrombosis) or overdose (and the ensuing increased risk of bleeding) (18). Regular contact with ‘warfarinised’ patients and the regular access to results of laboratory testing would engender some confidence in the clinicians in terms of their patient, including alleviating safety concerns. In contrast, as routine laboratory monitoring of DOACs is normally not required, clinicians may be less confident using DOACs, since there is limited reliable evidence about the ongoing anticoagulant effect of these drugs. Essentially, the ‘proof’ of their efficacy becomes the lack of any bleeding or thrombotic event in their patient whilst on a DOAC.
Regarding the consistent decrease of popularity of dabigatran compared to other DOACs, this may be at least partially attributable to the different mechanism of action (i.e., direct inhibition of thrombin versus FXa), which seemingly attributes a potentially higher anti-thrombotic potency to the former drug, but counterbalanced by a slightly enhanced risk of bleeding, especially of developing gastrointestinal hemorrhages (19,20). Last but not least, and unlike the other three DOACs, dabigatran is a prodrug, and its blood concentration is more dependent upon renal impairment (21). Due to the increasing global burden of chronic renal disease (i.e., ~10% for patients with chronic kidney disease at ≥ stage 3) (22), the impact of declining renal function on DOACs metabolism may also be seen as a reasonable explanation for the lower use of Dabigatran compared to the other three DOACs. As dabigatran was the only DOAC for a number of years to have a known antidote, it is somewhat surprising that its use is not favored in certain scenarios.
Finally, the still limited popularity of edoxaban in terms of Google searches is most likely attributable to the fact that this drug is the most recently approved agent among all oral anticoagulants, and its gradual worldwide dissemination will probably increase in parallel with approval by medical regulatory agencies and larger clinical use. For example, edoxaban is not yet approved for use in Australia, whilst it has only been recently approved in USA (in 2015) and Italy (in 2016).
Conclusions
The results of our analysis, based on Google searches for establishing the worldwide popularity of oral anticoagulant agents, reveals that warfarin remains the most popular oral anticoagulant around the globe and that rivaroxaban and apixaban are the second and third most popular, whilst the popularity of dabigatran appears very limited. Nevertheless, this situation will continue to change over time, and we would predict increasing popularity of DOACs, especially apixaban and rivaroxaban, but also increasing emergence of edoxaban, all at the cost of warfarin popularity. Increase in use of these new agents is also likely strengthened by the introduction of an effective antidote. It seems likely that the popularity of dabigatran may not return despite this agent being the first DOAC to have an antidote available for immediate reversal (23), inasmuch as an antidote has also become available for anti-FXa agents (24).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Blood for the series “Anticoagulant and antithrombotic therapy: globally applied according to local geographical selection criteria”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2018.12.04). The series “Anticoagulant and antithrombotic therapy: globally applied according to local geographical selection criteria” was commissioned by the editorial office without any funding or sponsorship. EJF served as an unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lippi G, Favaloro EJ. Laboratory hemostasis: from biology to the bench. Clin Chem Lab Med 2018;56:1035-45. [Crossref] [PubMed]
- Franchini M, Liumbruno GM, Bonfanti C, et al. The evolution of anticoagulant therapy. Blood Transfus 2016;14:175-84. [PubMed]
- Barnes GD, Ageno W, Ansell J, et al. Recommendation on the nomenclature for oral anticoagulants: communication from the SSC of the ISTH J Thromb Haemost 2015;13:2132-3. reply. [Crossref] [PubMed]
- Favaloro EJ, Pasalic L, Curnow J, et al. Laboratory Monitoring or Measurement of Direct Oral Anticoagulants (DOACs): Advantages, Limitations and Future Challenges. Curr Drug Metab 2017;18:598-608. [Crossref] [PubMed]
- Gosselin RC, Adcock DM, Bates SM, et al. International Council for Standardization in Haematology (ICSH) Recommendations for Laboratory Measurement of Direct Oral Anticoagulants. Thromb Haemost 2018;118:437-50. [Crossref] [PubMed]
- Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Emerg Med 2016;34:3-8. [Crossref] [PubMed]
- Cervellin G, Benatti M, Bonfanti L, et al. Quality and safety issues of direct oral anticoagulants in the emergency department. Semin Thromb Hemost 2015;41:348-54. [Crossref] [PubMed]
- Gosselin RC, Adcock DM. The laboratory's 2015 perspective on direct oral anticoagulant testing. J Thromb Haemost 2016;14:886-93. [Crossref] [PubMed]
- Kristoufek L. Can Google Trends search queries contribute to risk diversification? Sci Rep 2013;3:2713. [Crossref] [PubMed]
- Cervellin G, Comelli I, Lippi G. Is Google Trends a reliable tool for digital epidemiology? Insights from different clinical settings. J Epidemiol Glob Health 2017;7:185-9. [Crossref] [PubMed]
- Lippi G, Mattiuzzi C, Cervellin G, et al. Direct oral anticoagulants: analysis of worldwide use and popularity using Google Trends. Ann Transl Med 2017;5:322. [Crossref] [PubMed]
- Favaloro EJ, Pasalic L, Lippi G. Replacing warfarin therapy with the newer direct oral anticoagulants, or simply a growth in anticoagulation therapy? Implications for pathology testing. Pathology 2017;49:639-43. [Crossref] [PubMed]
- Whitworth MM, Haase KK, Fike DS, et al. Utilization and prescribing patterns of direct oral anticoagulants. Int J Gen Med 2017;10:87-94. [Crossref] [PubMed]
- Burn J, Pirmohamed M. Direct oral anticoagulants versus warfarin: is new always better than the old? Open Heart 2018;5:e000712 [Crossref] [PubMed]
- Favaloro EJ. Danger of false negative (exclusion) or false positive (diagnosis) for ‘congenital thrombophilia’ in the age of anticoagulants. Clin Chem Lab Med 2018; [Epub ahead of print]. [Crossref]
- Moroni F, Masotti L, Vannucchi V, et al. Confidence in the Use of Direct Oral Anticoagulants in the Acute Phase of Nonvalvular Atrial Fibrillation-Related Ischemic Stroke Over the Years: A Real-World Single-Center Study. J Stroke Cerebrovasc Dis 2018;27:76-82. [Crossref] [PubMed]
- El-Bardissy A, Elewa H, Mohammed S, et al. A Survey on the Awareness and Attitude of Physicians on Direct Oral Anticoagulants in Qatar. Clin Appl Thromb Hemost 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Favaloro EJ. How to Generate a More Accurate Laboratory-Based International Normalized Ratio: Solutions to Obtaining or Verifying the Mean Normal Prothrombin Time and International Sensitivity Index. Semin Thromb Hemost 2018. [Epub ahead of print].
- Skjøth F, Larsen TB, Rasmussen LH, et al. Efficacy and safety of edoxaban in comparison with dabigatran, rivaroxaban and apixaban for stroke prevention in atrial fibrillation. An indirect comparison analysis. Thromb Haemost 2014;111:981-8. [Crossref] [PubMed]
- Ntaios G, Papavasileiou V, Makaritsis K, et al. Real-World Setting Comparison of Nonvitamin-K Antagonist Oral Anticoagulants Versus Vitamin-K Antagonists for Stroke Prevention in Atrial Fibrillation: A Systematic Review and Meta-Analysis. Stroke 2017;48:2494-503. [Crossref] [PubMed]
- Lippi G, Favaloro EJ, Mattiuzzi C. Combined administration of antibiotics and direct oral anticoagulants: a renewed indication for laboratory monitoring? Semin Thromb Hemost 2014;40:756-65. [Crossref] [PubMed]
- Hill NR, Fatoba ST, Oke JL, et al. Global Prevalence of Chronic Kidney Disease - A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0158765 [Crossref] [PubMed]
- Facchinetti R, DeGuidi G, Pitoni F, et al. Rapid and well tolerated action of idarucizumab for antagonizing dabigatran in a patient needing urgent thrombolysis: a case report. Blood Coagul Fibrinolysis 2017;28:576-79. [Crossref] [PubMed]
- Lippi G, Sanchis-Gomar F, Favaloro EJ. Andexanet: Effectively Reversing Anticoagulation. Trends Pharmacol Sci 2016;37:413-4. [Crossref] [PubMed]
Cite this article as: Lippi G, Mattiuzzi C, Adcock D, Favaloro EJ. Oral anticoagulants around the world: an updated state-of-the art analysis. Ann Blood 2018;3:49.