The organization of transfusion and fractionation in France and its regulation
Introduction
Blood and blood components (whole blood, red blood cells, platelets, and plasma for transfusion) are classified by the World Health Organization (WHO) as “essential medicines” as they must meet the priority health care needs of a population (1). With due regard to public health relevance, evidence on efficacy and safety, and comparative cost-effectiveness, essential medicines should be available continuously in the context of operational health systems, at all times in sufficient quantity, in the appropriate dosage forms, with assured quality and adequate information, and affordability at the individual and community levels.
Every year, millions of patients are transfused with blood, blood components, or infused with plasma derivatives to improve their quality of life and survival. In 2012, European Member States (MS) reported that more than 1,350 blood establishments collect more than 20 million donations of whole blood and blood components (red blood cells, plasma, or platelets) as well as millions of additional donations. In France, approximately 500,000 people are treated every year with blood products.
Blood donations and PDMPs
Plasma for fractionation (PfF), the starting material of medicinal products derived from human plasma (PDMPs), is obtained from two types of donations; i.e., recovered from the whole blood donation or directly collected by apheresis. Apheresis plasma is used for transfusion or as starting material in the pharmaceutical sector to produce PDMPs.
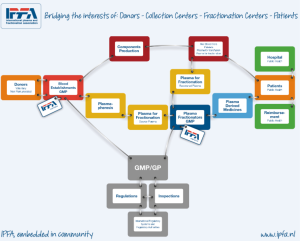
Figure 1 illustrates the “Plasma Chain” as described by the International Plasma and Fractionation Association (IPFA), of which the Laboratoire français du Fractionnement et des Biotechnologies (LFB) is a member organisation.
Among all PDMPs available in the world (2), coagulation Factor VIII, Factor IX, immunoglobulins, anti-D IgG, anti-tetanus IgG, and anti-rabies IgG are on the WHO Model List of “Essential Medicines”.
As this donated raw material is not available from other sources, PDMPs are products with strong added value. Some plasma proteins are, however, also produced by genetic recombination (e.g., coagulation factors).
The organization of transfusion and fractionation in France
The laws
In 1992, following the infection of haemophiliacs and other patients with HIV and HCV, the French Ministry of Health elaborated a new reform of the transfusion system that led to the Law 93–5 of 04 January 1993 (3) on the safety of blood transfusions and medicines. This law provides for a radical reorganization of blood transfusion institutions and regulations in France and separates the independent regulatory authorities from qualified operators.
The first step laid down by this law was the reorganisation of the National and Regional Blood Transfusion Centres, splitting activities of transfusion and fractionation by the creation of two separate entities. The French Blood Agency (AFS, “Agence Française du Sang”) appeared as the regulatory and national coordination body of the blood transfusion activities with the establishment of a new territorial organisation of the transfusion sector. In parallel, the fractionation activity of the centres of Lille, Lyon, Bordeaux, Strasbourg, Montpellier and Les Ulis were unified into a “public interest group” (“Groupement d’Intérêt Public”) the new LFB. This law prescribed that only LFB can prepare PDMPs from the blood or its components collected by the French blood establishments (4). At the same time, an independent regulatory authority was created, the French Medicines Agency (“Agence du Médicament”).
In addition, this law lays down the ethical principles of blood donation and labile blood products: “Blood transfusion is done in the interest of the recipient and is based on the ethical principles of volunteering and anonymity of the gift, and the absence of profit, under the conditions defined by this book.” (5). The donation is also only taken with the consent of the donor.
The law has also led to the development and application by transfusion institutions of “Good Transfusion Practices” to improve blood transfusion safety and to allow for the development and implementation of a national network for monitoring the collection and side effects of transfusions observed in recipients. “Haemovigilance”, a term derived from pharmacovigilance and applied to transfusion components, was created. France, a pioneer of haemovigilance (6) within Europe, was the first nation to introduce, by this law, a mandatory notification system as an important instrument for quality assurance. In November 1998, the European Haemovigilance Network (EHN) was founded with Luxembourg, Belgium, the Netherlands, Portugal, Switzerland, and France which was further developed in 2009 into the International Haemovigilance Network (IHN). In addition, in France, the Decree 95–566 of May 6, 1995 (7) established the pharmacovigilance of PDMPs, their traceability, and nominal dispensing.
A second law (Law 98–535 of July 1st, 1998) “on the strengthening of health surveillance and the control of the safety of products intended for humans” reorganises the public service for transfusion and replaces the regulatory body, the “Agence du Médicament” with the Health Safety French Agency for Sanitary Safety of Health Products (AFSSaPS), which now becomes the sole regulatory body (8).
The replacement of the French Blood Agency as provided by this law allowed the separation of activities formerly devolved to this organisation and established the single blood transfusion operator: the French Blood Establishment (Établissement Français du Sang, EFS), for the operational activities, while regulatory and normative activities were transferred to AFSSaPS. Accordingly the EFS mission became the following: monopoly collection of donations of blood and plasma on the national territory; preparation of blood products, biological qualification of donations, and distribution of blood products; provision of health care facilities with labile blood products; assuring the public service of blood transfusion; and guarantying national self-sufficiency in blood products. In addition EFS became the exclusive supplier of PfF to LFB for the manufacture of PDMPs performed in compliance with the Good Transfusion Practices that govern each stage of the transfusion including sampling, preparation, qualification, distribution, and transportation.
Subsequently, a new directive established in 2016, the Good Practice Guidelines (9) (GPG), requires European Blood Establishments to take fully into account the standards and specifications set out in this directive when implementing their quality system.
Composed of a national headquarter and 15 regional establishments (12 in metropolitan France and 3 in French overseas departments), EFS with its 9,730 collaborators has been collecting blood or plasma from 1,697,460 donors throughout France in 2016, processing and testing their donations in 4 production centres and has supplied blood products to more than 1,500 healthcare facilities (e.g., hospitals and clinics) in France. The EFS is present throughout the country with 132 fixed collection sites and 40,000 mobile collections organized each year.
Three types of donation are collected at EFS: whole blood, plasma and platelets. Most of the plasma is collected by apheresis. Ninety percent of the plasma collected is dedicated for the manufacture of PDMPs and the remaining 10% is used for “therapeutic” transfusions.
In parallel, the organization that was set up to meet the medical needs and especially for blood during the war time is still present today. The military blood transfusion centre (CTSA; Centre de Transfusion Sanguine des Armées) is a particular organization for the military which is independent from the EFS. In 2005, an agreement was signed between the CTSA and the EFS to harmonize blood collection practices and to give priority to the CTSA for military sites. The blood collected and prepared by the CTSA is for the benefit of the armed forces in operations abroad and in military hospitals.
In addition to the creation of AFSSaPS, Law 98–535 provided for the creation of several additional structures which include the National Committee of Sanitary Security (CNSS), the Institute of Health Surveillance (InVS) and the National Institute of Blood Transfusion (INTS).
- The CNSS is responsible for analysing events likely to affect the health of the population, to process the available information, and to ensure the coordination of the scientific policy of InVS and AFSSaPS;
- The InVS acts as a reference centre and provides a constant surveillance of the health status of the population. As a public institution, placed under the supervision of the Ministry of Health, InVS brings together surveillance, vigilance, and alert missions in all areas of public health. InVS has seen its missions reinforced by Law 2004-806 of 9 August 2004 (10) on public health policy, in order to respond to the new challenges revealed by recent health crises and emerging risks;
- Research and training are the reference activities of INTS with a view to contributing to the improvement of transfusion safety, the prevention of risks, and the adaptation of transfusion activity to technical and scientific developments. It is also responsible for providing, on request, to the Ministry of Health and other National Agencies (Public Health, AIDs research and HTA), all information relating to the blood transfusion sector in its field of competence (11). In May 2017, a plan to redistribute the mission of INTS has been set up with the creation of the High Council of Blood Transfusion.
On 01 February 2006 the French Decree 2006-99 “on EFS and haemovigilance amending the Public Health Code (regulatory provisions)” specified and reinforced the haemovigilance procedures in particular.
On 01 May 2012, by the Law 2011–2012 of 29 December 2011 (12) “on strengthening the safety of medicines and health products”, the National Agency for the Safety of Medicines and Health Products (ANSM) replaced AFSSaPS when it took over their missions, rights, and obligations. It was accordingly endowed with new responsibilities and missions, powers, and strengthened authority. ANSM now covers all health products.
In addition, this law sets new requirements for ANSM for transparency (traceability of work that precedes decision-making, recording of meetings, publication of minutes with expression of minority opinions), independence of experts involved in the works of the Agency (public declaration of interest, ethics commission, etc.), and the sharing of information with all audiences (health professionals, patients, and the general public).
ANSM is a public institution placed under the supervision of the Ministry of Health, financed by a subsidy for public service charge received from the State, of an amount of € 115M in 2015. ANSM is led by both the Director General and its management board. The Director General is a delegate of the State exercising power with regard to human and animal health protection and related decisions on behalf of the State. The Director General is also the executive body of the institution for the organization, management, and general policy activities of the Agency, taking decisions on behalf of the institution.
LFB is a special actor of the French market for PDMPs
LFB is an industrial player in the health care sector. It is the leader in the French market for PDMPs and a public limited company with majority public capital, the State is its main shareholder.
LFB is required to fulfil a public health mission in France under Law 2009-879 of July 21st, 2009—article 77, modifying the article L5124-14 of the Code of Public Health as follows: it “Fractionates in priority the plasma from blood or its components collected by the EFS to meet national needs, particularly those related to the treatment of rare diseases. It distributes, primarily on French territory, the medicines that come from it.” (13). LFB, in return for its exclusive access to EFS voluntary donated plasma collected on French territory, gives priority to national needs, treating more than 500,000 patients each year in France. Due to this national mission, LFB has positioned himself as a fractionator with one of the largest portfolios of 23 PDMPs (14).
LFB may also fractionate plasma other than that provided by EFS for its international supply in PDMPs and most of its products are marketed in more than 40 countries. In addition, LFB is entitled to perform contract fractionation activities for other countries.
LFB employs approximately 2,000 people across 5 bioproduction plants, of which 4 are located in France: two in Ulis, one in Lille, and one in Alès. With major industrial investments in Lille and Les Ulis, LFB is aiming to triple its capacity with a future site in Arras in Northern France.
LFB also carries out research and production activities on drugs that can replace blood products. Of the €502.4 M in turnover, 18% is invested in R & D; 30% of turnover derives from international business with a more than 90% increase from these international sales in the last 5 years. In addition to PDMPs, LFB is one of the few manufacturers in the world to master two additional highly specialised and promising areas in biopharmaceuticals: (I) the development of recombinant therapeutic proteins and monoclonal antibodies and (II) manufacturing of advanced therapy medicines (cell therapy and gene therapy) in its “Cell for Cure” affiliate (a result of an important innovation policy).
LFB relies on trading partners for its international development and also on subsidiaries in Belgium, Germany, Spain, the United Kingdom, Brazil, Mexico, and the United States.
Rules governing blood and blood products in the European Union
The European regulation on blood and its components is divided into three daughter directives subsequent to Directive 2002/98/EC of the European Parliament and Council of 27 January 2003 “setting standards of quality and safety for the collection, testing, processing, storage and distribution of human blood and blood components and amending Directive 2001/83/EC” (15): Directive 2004/33/EC (16) “bringing new technical requirements for blood and blood components”; Directive 2005/61/EC (17) “giving new requirements for traceability and notification of adverse reactions and incidents”; and Directive 2005/62/EC (18) “concerning Community standards and specifications for a quality system in blood establishments”. This regulation constitutes a common foundation of quality and blood safety measures in the European Union and is guaranteed for all citizens circulating in the MSs of the Union. However, a state may maintain or establish more stringent requirements. These directives create a series of provisions for the eligibility of applicants for blood donation, collection, and biological qualification of donation and delivery of blood and its components.
Article 20 of Directive 2002/98/EC encourages voluntary and unpaid blood donations. The Directive also defines ‘haemovigilance’ as a set of organised surveillance procedures relating to serious adverse or unexpected events or to reactions not only in recipients, but also in donors, and to epidemiological follow-up of donors. Chapter V of this directive, haemovigilance (traceability, documentation, and notification of serious adverse events and reactions), also describes the requirements. It extended the scope of national systems for the reporting of serious incidents of the transfusion chain and serious adverse reactions in blood donors.
Pharmacovigilance is regulated by European Directive 2010/84/EU (19) and Regulation 1235/2010 (20) which aims to rationalize the decisions of the European Union on issues of drug safety and to guarantee the application of the measures to all drugs of the European Union. This directive specifies good pharmacovigilance practices (GPV).
European directives were transposed into national law between 2004 and 2007 by legislative and regulatory means.
The rules governing medicinal products in the European Union
Regulatory environment: International Council for Harmonisation’s of Technical Requirements for Pharmaceuticals for Human Use (ICH)
ICH brings together the regulatory authorities and pharmaceutical industry trade associations to discuss scientific and technical aspects of drug registration in order to achieve greater regulatory harmonisation worldwide. ICH codified guidelines to address quality (Q), safety (S), efficacy (E), and multidisciplinary (M) and can serve as reference for regional guidance. Standing regulatory members represent Europe (European Commission), Japan (HMLV, JPMA), USA (FDA/PhRMA), Canada (Health Canada), Switzerland (Swissmedic), Brazil (ANVISA), South Korea (MFDS) and China (CFDA), and also industry representatives (Bio, EPFIA, IGBA and WSM).
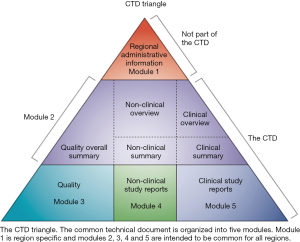
Among its guidelines, ICH has issued M4 on the agreement to assemble all the Quality, Safety and Efficacy information in a common format (the CTD, Common Technical Document) when seeking Marketing Authorisation (MA) for a medicinal product (Figure 2).
The European Commission
The European Commission has laid out the rules for MAs of medicinal products in its Eudralex relating to medicinal products for human use. Volume 1 (21) is dedicated to the pharmaceutical legislation (directive & regulation) while Volume 2 (22) is the notice to applicants and describes, in volume 2A, the rules for Procedures; in volume 2B, presentation and content of the MA Application (MAA) dossier; and in volume 2C, regulatory guidelines related to procedural and regulatory requirements. Volume 3 refers to all Guidelines issued by the European regulatory body.
The European Directive 89/381 (23) states that stable blood products prepared from human blood and plasma (i.e., PDMPs) become “medicinal products”. As laid down by Directive 75/318 modified by 91/507 (and later by Directive 2001/83/EC) these products are now subject to marketing authorization procedure with quality, safety, and efficacy criteria. Blood and blood components (labile products) are excluded from the scope of this directive.
As for any medicinal product, the submission of a PDMP for a MA should follow the requirements of procedure and dossier format defined by ICH. The European adjusted format of the CTD with each part at a specific place in its architecture is provided in Figure 2.
The European directorate for the quality of medicines (EDQM)
EDQM is one of the directorates of the council of Europe. It contributes directly to the quality and safety of blood, blood components, and PDMPs, as for other medicines. One important Department is in charge of the European Pharmacopoeia (24), which acts as a single reference for the quality control of medicines. The monographs and official standards provide a legal and scientific basis for quality control during the development, production, and marketing processes. They relate to the qualitative and quantitative composition and the tests to be carried out on medicines, on the raw materials used in production and on intermediate materials produced during synthesis or manufacture. All producers of medicines and/or substances for pharmaceutical use must therefore apply these quality standards in order to market their products in the signatory states of the convention.
Directives 2001/83/EC and 2001/82/EC, on medicines for human and veterinary use, respectively, emphasise mandatory compliance with European Pharmacopoeia monographs when requesting a MA.
In France, the French Pharmacopoeia fulfils the conditions of Article L 5112-1 of Law 2009-594 of 27 May 2009 and includes monographs of the overseas pharmacopoeia.
Directive 2012/26/EC of the European parliament and of the council amends Directive 2001/83/EC with regard to pharmacovigilance. It reinforces its organization by setting up a committee for pharmacovigilance risk assessment (PRAC) within the European Medicines Agency (EMA).
Regulatory environment: ANSM
ANSM, as mentioned above, evaluates the safety, efficacy, and quality of health products including medicinal products, medical devices, in-vitro diagnostics, biological products including labile blood products, as well as cellular and gene therapy products, organs, tissues, and cells used for therapeutic purposes, micro-organisms and toxins, ancillary therapeutic products, mother’s milk and biocidal, cosmetics, tattoo products and raw materials, before and after MAA, including for clinical trials.
ANSM has overall responsibility to ensure the enforcement of the laws and regulations relating to the import, testing, manufacture, preparation, export, wholesale distribution, packaging, preservation, operation, market access, advertising, and servicing of health products in France including blood products. ASNM makes safety decisions in the name of the Nation with policing powers to protect public health and guarantee safe use of health products (e.g., ban, suspension or restriction on a product or activity).
The Decision DG 2012-237 of 24 September 2012 on the organisation of the ANSM introduces in 2013 a matrix operation involving Métiers and Product Departments. The evaluation of applications for MAs is performed by dedicated product departments assisted by working groups of external experts and in conjunction with the Evaluation Departments. As the links between pre-authorisation and post-marketing surveillance with regard to safety are strengthened, the assessment is also done in conjunction with the Surveillance, Inspection, and Control Departments.
Blood and blood products and PDMPs, as well as medical devices and detection kits are assessed by the Department of Oncology, Haematology, Transplantation, Nephrology, Cell Therapy Products, Labile Tissues and Blood Products, assisted by the “Labile Blood Products and Blood Donors” expert working group for blood and blood products, and by different working groups according to the therapeutic area of the PDMPs assessed (e.g., oncology and haematology, neurology or viral infectious diseases working groups). Both types of products undergo an assessment by the “Viral and Microbiological Safety of Health Products” working group.
After assessment of blood and blood components, ANSM decides and establishes the list and characteristics of labile blood products, published by decree in the Official Journal of the French Republic. For PDMPs, ANSM either grants a French MA if following a national, mutual recognition, or a decentralised procedure, or participates in the centralised European procedure for a unique MA granted by the European Commission.
Regulatory environment: EMEA/EMA
The European Agency for the Evaluation of Medicinal Products (EMEA) was created in 1995 by way of article 71 of the council regulation (EEC) No 2309/93 of 22 July 1993 “laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Agency for the Evaluation of Medicinal Products”. In 2004, the Regulation (EC) No 726/2004 of the European Parliament and of the Council of 31 March 2004 established the EMA to improve the operation of procedures for the approval and marketing authorization of medicinal products in the community and to amend certain administrative aspects of the agency.
The agency currently coordinates scientific resources from the 28 MSs for the evaluation, supervision, and pharmacovigilance of medicinal products.
The agency comprises 7 scientific committees headed by the committee for medicinal product for human use (CHMP, formerly CPMP) which carries overall responsibility for the scientific opinions on quality, safety, and efficacy of the medicines for human use. It includes one representative for each MS, a chairman who is nominated for 3 years, and standing permanent working parties: the Healthcare professionals, the Biologics, the Patients and Consumers, Quality, the Safety, and the Scientific advice working parties. With an additional 11 temporary technical Working Parties they conduct the scientific work of the agency.
The Working Groups related to the PDMPs are the Blood Products Working Party (BPWP) for non-clinical and clinical development and the Biologics Working Party (BWP) for the quality development. Both the BPWP and BWP may solicit the help of specific ad-hoc working groups or subgroup meetings as needed.
Authorisation of blood and blood components is not in the scope of the EMA. However, the body of European inspectors supervises the inspections of worldwide plasma collection centres providing PfF for PDMPs marketed in Europe.
The EMA is also responsible for reviewing applications for orphan designation of medicines within the framework of the Orphan Regulation in Europe originally advocated by France. A dedicated unit within the French Ministry of Health developed a Memorandum in 1995 which was brought to the European Commission, resulting in the Regulation (EC) No 141/2000 (25) as instituted on 16 December 1999.
To qualify for orphan designation, a medicine must meet a number of criteria:
- It must be intended for the treatment, prevention, or diagnosis of a disease that is life-threatening or chronically debilitating;
- The prevalence of the condition in the EU must not be no more than 5 in 10,000 or it must be unlikely that marketing of the medicine would generate sufficient returns to justify the investment needed for its development;
- No satisfactory method of diagnosis, prevention, or treatment of the condition concerned exists, or, if such a method does exist, the medicine must be of significant benefit to those affected by the condition.
Applications for orphan designation are examined by the EMA’s Committee for Orphan Medicinal Products (COMP), using the network of experts that the committee has developed. After a positive COMP opinion, the European Commission grants the orphan designation and adds it to the full list of orphan designations available in the community register of orphan medicinal products for human use (26).
Because of Brexit, UK will leave the European environment by March 2019 and EMA will be relocated from London to Amsterdam (if not Milan, as Italy appealed against the decision to relocate EMA). Depending of the results of the negotiations between UK government and the European Commission, a number of changes will have to take place, both for the agencies and for the applicants.
Assessment by EMA
Quality
Registration procedures vary depending of the choice of the applicant and other considerations:
- The centralised procedure leads to the evaluation by EMA and a unique European MA granted by the European Commission valid in the 28 MS; this procedure is mandatory for biotechnological and orphan products;
- If a product is already licensed by one MS, the national MA can be mutually recognised by various MS chosen by the applicant;
- When the product has never been authorised in any MS, and the applicant applies for MA in some MS, the procedure is decentralised among these MS.
As PDMPs are not prepared using biotechnological methods (except for those purified by monoclonal antibody affinity chromatography), they are not submitted to the mandatory centralised registration procedure; rather, it is the choice of the applicant to go through one or the other European registration procedures.
To help applicants develop human medicines and prepare MAA, the CHMP publishes scientific reference guidelines which reflect a harmonised approach of the European Agency and MS on how to interpret and apply the requirements for quality, safety and efficacy set out in the community directives and including ICH guidance. Adopted guidelines are complementary to European Pharmacopoeia monographs and chapters (27-29). These guidelines replace the previous volume 3 of the Rules governing medicinal products in the EudraLex (30), published by the European Commission.
EMA quality guidelines are provided by the Quality Working Party for all medicinal products on all aspects of starting material, manufacturing, and product (including active substance, manufacturing, impurities, specifications, analytical procedures and analytical validation, excipients, packaging, stability, pharmaceutical development, life cycle management, quality by design and post-approval change management protocols). For biological medicines and materials of animal and/or human origin, the BWP also provides specific requirements including those for investigational biological medicinal products in clinical trials.
While PDMPs must follow all requirements for any medicinal product, special guidelines have been written for their development, both for the active substance (with plasma as the starting material) and the finished product.
These guidelines are integrated for quality control assessment of raw and starting materials, all of which fall under the requirement for good manufacturing practices.
Viral safety
Special attention is required to address the viral safety of any product of human or animal origin, such as PDMPs, whether used as an active ingredient, an excipient, or during the manufacture of a product.
Biological safety needs to be built, assessed, and monitored. PDMP safety is built on application of a progressive set of measures from the donor to the patient on 3 levels: (I) selection of donors on epidemiological criteria and risk factors; (II) testing and control of the starting material(s); and (III) manufacturing methods including validated steps for viral inactivation/removal and controls at different stages. None of these safety measures is by itself enough; the contribution of each brings an additive margin of safety.
LFB is responsible for introducing dedicated viral inactivation/elimination steps in the manufacturing processes of PDMPs, application of quality control tests to Products and in-process materials, and for compliance with GMP to ensure batch consistency and freedom from product defects or contamination.
Responsibility for evaluation also lies with health authorities including the ANSM through the creation of appropriate regulatory frameworks, application of strict regulatory procedures and assessment of the whole biological safety process in line with European or ICH Notes for Guidance.
The requirement for viral safety of both PDMPs and blood components has led to technological advances in PDMP manufacture such as solvent/detergent treatment, nanofiltration, heat treatment and large scale pathogen screening of the starting plasma using sensitive Nucleic Acid Testing methods. These technologies have contributed to the current excellent safety record of PDMPs. In addition pathogen inactivation (PI) by either Psoralen-Amotosalen + UVA, Methylene Blue, or Riboflavin/UV treatment have been applied to blood components to further minimise the risk of pathogen contamination in such products. Whilst these PI processes are not required for blood components by current regulations their application offers future prospects for further reducing infection by known and emerging pathogens which escape detection by current test regimes. The PI could remove the need for individual pathogen detection.
The EMA BWP guidelines on viral safety address all stages along the preparation of PDMPs and take into account the WHO biological safety, namely:
- Virus validation studies: the design, contribution and interpretation of studies validating the inactivation and removal of viruses (31);
- Warning on transmissible agents in summary of product characteristics and package leaflets for PDMPs (32);
- Virus safety evaluation of biotechnological investigational medicinal products (33).
The manufacturing process of PDMPs must include orthogonal inactivation/removal steps that are able to act on a wide range of viruses of diverse physico-chemical characteristics which must be compatible with the “resistance” of the protein product to be prepared to limit the risks of protein denaturation or neo-antigen formation. The safety steps are validated by laboratory studies assessing the ability to remove or inactivate viral infectious agents, and defining log reduction factors, and to foster the relationship between the reduction factors and the residual risk for the different agents. The EMA/CHMP/BWP/706271/2010 guideline specifies that “…it is desirable in most cases to incorporate 2 distinct effective steps which complement each other in their mode of action [ ]; at least one of the steps should be effective against non-enveloped viruses.”
The BWP has also issued a reflection paper on viral safety of PDMPs with respect to hepatitis E virus (34) and a Position statement on the safety with regards to West Nile virus and PDMPs (35).
Improving the control of the viral safety of PDMPs is an ongoing process that must evolve with the state of knowledge and requires permanent scientific surveillance.
Re-examination of processes is necessary to ensure a sufficient level of viral safety. The introduction of a new test or method does not necessarily mean that batches previously produced are not safe and the pharmacovigilance data obtained in the treated patients represent the ultimate proof of viral safety of the product. No transmission of viral disease has been observed for the last 25 years as products are subjected to very efficient virus inactivation and removal processes.
Prion diseases: transmissible spongiform encephalopathies (TSEs) regulations
As a consequence of the bovine spongiform encephalopathy contamination of cattle, the risk of transmission of spongiform encephalopathies and particularly of the variant form of Creutzfeldt-Jakob disease (vCJD), by blood and blood products, and theoretically by PDMPs, has led to the application of the precautionary principle with worst case scenario risk assessments and the implementation of several safety measures in the UK, France, and Europe. To date, there has been no transmission of vCJD by PDMPs or by leucocyte depleted blood products (36,37).
In addition to risk analyses, dedicated reduction steps are introduced in the manufacturing process. AFSSaPS performed vCJD risk analyses from 2000 to 2009 because of the number of clinical cases in the country.
In 2000, AFSSaPS recommended full introduction of leucoreduction for blood components and accelerated implementation by LFB of the 15nm nanofiltration for its FVIII product. The risk exposure for each product was calculated based on epidemiology of vCJD, infectivity by plasma pool, infectivity/fraction, quantity of active substance produced, infectivity per IU of active substance, administered dose per patient and per year, reduction factors due to precautionary measures as donor selection, leucoreduction and manufacturing process, leading to theoretical residual infectivity of 10−7.18 to 10−0.12 (log infectious units IV/annual dose/patient), that is for example, a risk of transmission per 103 injections of FVIII per year (38).
In June 2011, EMA published its position statement (39) that asserted the efficiency of prion removal by PDMP manufacturing processes in tested models, recommending precautionary measures such as donor selection (UK residence), leucoreduction, processes (including nanofiltration) and permanent ongoing surveillance by scientists, operators, and authorities. This position statement is still due to be revised and published in 2018. EMA assessment of PDMPs of safety vis-à-vis TSEs is informed by a number of further BWP guidelines (40,41), questions and answers (42), position papers, public statements, and reports (43,44).
With regards to classical CJD, while the EMA Position paper reads: “Cumulative epidemiological evidence does not support transmission of sporadic, genetic and iatrogenic Creutzfeldt-Jakob disease (CJD) by PDMPs. There is no change to the previous CHMP position that recall of PDMPs is not justified where a donor is later confirmed as having sporadic, genetic or iatrogenic CJD.”, France had imposed batch recall measures until December 2015 but adopted the EMA recommendation thereafter.
Plasma master file (PMF), quality and biological safety
The concept of PMF was established via European legislation in June 2003 after proposals from the agencies including AFSSaPS. This concept was created in order to simplify registration processes by submitting and assessing only once the starting material documentation for all identified plasma sources of PDMPs and for all the countries where they are registered. This initiative removed the need for submission of plasma raw material safety and quality data for individual MA applications
The PMF is a compilation of not only all the required scientific data on the quality and safety of human plasma used in their manufacture, medical devices, and experimental products, but also all information on the technical information on this starting material. These data cover all aspects of plasma collection and supply, from collection to the plasma pool, start of the fractionation manufacturing process, as well as contractual agreements between the plasma collectors and the fractionators.
The PMF is a stand-alone document separated from the MAA file. The structure and scientific data are described in the EMA guidelines on the scientific data requirements for a PMF (45), on the requirements for PMF certification (46), on the epidemiological data on blood transmissible infections (47), and in two guidelines on validation of immunoassay for the detection of antibody to human immunodeficiency virus and of hepatitis B virus surface antigen in plasma pools (48,49).
The principles of a certificate of conformity with community legislation of the PMF issued by the EMA through a centralized procedure are described in the Note Procedural Guidance on PMF (50).The PMF is again evaluated in a second step by the relevant MS in the context of a MAA assessment, which appears to contradict the PMF concept by creating unnecessary costs associated with assessment and submission.
Regulation of clinical safety and efficacy of PDMPs
EMA clinical efficacy and safety guidelines for PDMPs (including biotechnological alternatives) are provided by the BPWP (51). Guidelines on the clinical investigation of most PDMPs are available (52). Guidelines for the text of the Core Summary of Product Characteristics including a general guideline on the warning on transmissible agents are also published. These guidelines are “not binding”; however, they undoubtedly set the reference point for the assessment of the MAA files, by both EMA and individual MS competent authorities. For each of the PDMPs (or recombinant product), the clinical investigation guidelines distinguish between the requirements for a new product and for a product with changes in its manufacturing process, although for the most part, the difference is not so significant in terms of burden of development.
ANSM, together with most of the MS competent authorities, follows the clinical guidelines published by the BPWP in its assessments.
For the development of any medicinal product for use in the paediatric, a paediatric investigation plan (PIP) should be submitted to the Paediatric Committee (PDCO) for validation, according to the paediatric regulation (53). A MA cannot be granted without the agreement of the PIP. Interestingly, with regards to paediatrics for FVIII, FIX, and immunoglobulins, instead of following the regular path, the BPWP sets its own rules in its clinical investigation guidelines.
The issue of inhibitor development in patients receiving FVIII is poorly addressed in these guidelines. The guidelines state that intrinsic FVIII immunogenicity should be studied in patients who have received >150 exposure days of the product when the immune system is already adjusted to the new molecule. In fact, intrinsic immunogenicity is revealed by the first infusions of the product in patients previously untreated. These FVIII guidelines are currently under revision (54).
In light of increasingly stronger links between pre-MA and post-marketing assessment, safety issues are dealt with by the Pharmacovigilance Risk Assessment Committee (PRAC) which can reassess products if a safety issue is raised. This was the case for the incidence of inhibitors in previously untreated patients by FVIII when the Sippet Study demonstrated a 1.83 higher risk of evaluated recombinant versus plasma-derived FVIII products (55). Surprisingly, the PRAC conclusions are not aligned with those of the scientific community, such as the Rodin (56) or the FranceCoag (57) studies that clearly evidence a higher risk of generation of inhibitors by recombinant FVIII. The PRAC recommendations, endorsed by EMA and the European Commission, have led to changes in the Core SmPC of the relevant products, attaching little importance to the role of the differences in molecular nature of the products with regard to immunogenicity. PRAC also has recently recommended the suspension of the MA of hydroxyethyl-starch solutions for infusion across the European Union following studies showing low adherence to restrictions aimed at reducing risks of kidney injury and death (58).
Batch release
The batch release of immunological and blood derivatives instituted by the European Commission (59) to strengthen the protection of public health is an official pre-marketing control of individual product batches and acts independently of the control and release of batches by the manufacturer’s qualified person. This procedure aims at checking the conformity of a batch of PDMPs to the specifications of relevant European Pharmacopoeias and to the MAA file. It is carried out on the manufacturing plasma batch and on the finished product for some viral markers and potency and other quality parameters by one of the national Official Medicines Control Laboratories (OMCL) of the European Economic Area at the request of a MS. The batch release certificate issued by one OMCL is recognized by all other MS.
The European Pharmacopoeia published a general explanatory note “Control authority batch release of vaccines and blood products” (60) and specific explanatory notes to human albumin, coagulation factors, plasma inhibitor concentrates, human plasma (pooled and treated for virus inactivation), biological fibrin sealants, and human immunoglobulins (61).
PDMPs securing chain
Traceability from the donor to the patient and from the patient to the donor is a mandatory element of control to ensure product quality and donor/patient health. It is both a descending traceability (from donor to recipients, including distribution) performed through haemovigilance plasma providers, and an ascending traceability (from recipients to donors) through pharmacovigilance in hospitals, health care professionals, or patients. The plasma collectors ensure screening of donors through a medical questionnaire and exam as well as viral testing for single donations. Should a donor subsequently test positive for a viral marker or new information becomes available regarding a donor’s health status, information is forwarded to the fractionator.
If the information is available during the plasma quarantine period held by the fractionator, the donation is destroyed prior to pooling for fractionation via a lookback process. In case the information is generated after manufacturing, plasma product batches manufactured with this specific donation can be recalled from the market by the fractionator after conducting a risk assessment and following agreement with the competent authority.
Information could also originate from a recipient of blood and blood component pharmacovigilance notification, raising concerns for the quality or safety of the donation. The lookback procedure will identify the donation included in a plasma pool for fractionation. By identifying each donation contributing to the plasma pool, the donor can be informed of any required health and medical measures to be taken in the case of donor infection.
PDMP safety in the French organisation, a set of measures from donor to patient
In France, post-donation and post-transfusion data are collected on 1.8 million donors, 2.6 million donations, and for 18 PDMPs administered to about 500,000 patients per year. EFS produces and delivers approximately 3,000,000 PfF bags every year.
EFS is responsible for the production of the starting plasma material, either from whole blood or plasmapheresis, by ensuring the selection of the unpaid blood donors with regard to compliance with donation and exclusion criteria (including any risk behaviours), interviews, and medical examination of the donors. EFS performs individual viral testing and minipool testing of blood donations on indirect markers (anti-HIV 1+2, anti-HCV, anti-HBc and anti-HTLV I + II antibodies) and direct markers (HBs Ag, PCR control for HIV-1 RNA, HCV and HBV DNA).
LFB first applies a quarantine period to allow management of post-donation information provided by EFS, if any. Upon PfF receipt, LFB performs PCR testing on minipools to check Parvovirus B19 and HAV, and virological control of manufacturing plasma pool (serology and PCR): anti HIV-1+2 ab and HIV-1 RNA, HCV RNA (≤100 UI/mL), HBsAg, HBV DNA, HAV RNA and B19 DNA, according to the European Pharmacopeia of PfF (62,63).
Despite their transposition into French law, ANSM imposes for PfF some testing not required by the European Blood technical directives. This demonstrates the current freedom of MSs to adapt the regulation rather than accepting the level of European requirements.
Radio frequency identification technology used in France by EFS and LFB, allows, through a complex record keeping system, identification and traceability of plasma from each donation and each donor to the plasma pool for manufacturing and through to the finished batch of PDMPs. LFB also performs quality assurance audits at EFS to ensure it complies with the specification of the plasma quality agreement signed between the two organisations. Full traceability is met by all stakeholders, either EFS or other plasma providers, LFB and ANSM.
Conclusions
Like many MS of the EU the regulation of Blood Products and PDMPs in France has evolved from a system of ‘self-regulation’ until the early 1990’s to today’s full, comprehensive and independently accountable activity. The experiences of the past require that it should be so. The regulatory framework and expertise necessary to assure the safety of patients in France is today well established and actively contributes to and informs the continuous review of EU wide policies, guidelines and directives.
The European Commission has started to reflect on Substances for Human Origin regulations as a whole (64) and the protection of the human body and its parts (65). The aim is to identify common standards and principles where appropriate and harmonisation where possible among the entire sector. However, the landscape of blood, blood products and PDMPs remains complex and under scrutiny. One of the possible outcomes of the European Regulation revision might be that MS uniformly transpose without deviation the European rules. Another possible outcome could be that EMA or another newly created European Agency takes the responsibility for blood and blood products evaluation and supervision. This would help assessing the starting materials for PDMPs (i.e., plasma recovered from whole blood donation or obtained by apheresis). Haemovigilance could also then be supervised by a European authority.
Acknowledgments
The author thanks Thierry Burnouf and Robert Perry for their English editing assistance.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Thierry Burnouf) for the series “Plasma Fractionation” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: The series “Plasma Fractionation” was commissioned by the editorial office without any funding or sponsorship. Françoise Rossi is a salaried employee of LFB.
Ethical Statement: The author is accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO Model List of Essential Medicines 19th List April 2015. Available online: http://www.who.int/medicines/publications/essentialmedicines/EML_2015_FINAL_amended_NOV2015.pdf
- WHO recommendations for the production, control and regulation of human plasma for fractionation. Available online: http://www.who.int/biologicals/publications/ECBS%202005%20Annex%204%20Human%20Plasma%20Fractionation.pdf
- Loi n° 93-5 du 4 janvier 1993 relative à la sécurité en matière de transfusion sanguine et de médicament. Available online: https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000000178299
- Code de la santé publique - art. L670-2 (M). Available online: https://www.legifrance.gouv.fr/affichCodeArticle.do?cidTexte=LEGITEXT000006072665&idArticle=LEGIARTI000006694292
- Code de la santé publique - art. L666-1 (M). Available online:https://www.legifrance.gouv.fr/affichCodeArticle.do?cidTexte=LEGITEXT000006072665&idArticle=LEGIARTI000006694163
- Code de la santé publique - Article L1221-3 | Legifrance – Légifrance; Code de la santé publique - Article D1221-7 | Legifrance - Légifrance. Available online: https://www.legifrance.gouv.fr/affichCodeArticle.do?cidTexte=LEGITEXT000006072665&idArticle=LEGIARTI000006686077&dateTexte=&categorieLien=cid; https://www.legifrance.gouv.fr/affichCodeArticle.do?cidTexte=LEGITEXT000006072665&idArticle=LEGIARTI000006908754&dateTexte=&categorieLien=cid
- Décret no 95-566 du 6 mai 1995 relatif à la pharmacovigilance exercée sur les médicaments dérivés du sang humain et modifiant le code de la santé publique (deuxième partie: Décrets en Conseil d'Etat). Available online: https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000000371630&categorieLien=id
- Décret no 99-142 du 4 mars 1999 relatif à l'Agence française de sécurité sanitaire des produits de santé. Available online: https://www.legifrance.gouv.fr/affichTexteArticle.do;jsessionid=1C3FD9BDC5D5CB905188DD9926AD9DBA.tplgfr21s_3?cidTexte=JORFTEXT000000758692&idArticle=LEGIARTI000006723496&dateTexte=20180512&categorieLien=id
- Directive (EU) 2016/1214 of 25 July 2016 amending Directive 005/62/EC as regards quality system standards and specifications for blood establishments. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016L1214&from=FR
- Law 2004-806 of 9 August 2004. Available online: http://data.europa.eu/eli/dir/2016/1214/oj
-
Accueil - Institut National de la Transfusion Sanguine (INTS) - LOI n° 2011-2012 du 29 décembre 2011 relative au renforcement de la sécurité sanitaire du médicament et des produits de santé. Available online: https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000025053440&categorieLien=id
- LOI n°2015-990 du 6 août 2015 – Article 190, modifying article L5124-14 of the Public Health Code. Available online: https://www.legifrance.gouv.fr/affichCodeArticle.do?cidTexte=LEGITEXT000006072665&idArticle=LEGIARTI000031012558&dateTexte=&categorieLien=id
-
Mission LFB - Directive 2002/98/EC of the European Parliament and of the Council of 27 January 2003. Available online: https://ec.europa.eu/health//sites/health/files/files/eudralex/vol-1/dir_2002_98/dir_2002_98_en.pdf
- Directive 2004/33/EC of 22 March 2004 implementing Directive 2002/98/EC. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/10/WC500004484.pdf
- Directive 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/10/WC500004485.pdf
- Directive 2005/62/EC of 30 September 2005 implementing Directive 2002/98/EC. Available online: https://www.edqm.eu/medias/fichiers/directive_200562ec_of_30_september_2005_implementing_directive_200298ec_of_the_european_parliament_a.pdf
- Directive 2010/84/EU - European Commission. Available online: https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-1/dir_2010_84/dir_2010_84_en.pdf
- Regulation EC 1235/2010. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwjhm_TNx57dAhVJ2qQKHV2GAtQQFjAAegQIABAC&url=https%3A%2F%2Feur-lex.europa.eu%2FLexUriServ%2FLexUriServ.do%3Furi%3DOJ%3AL%3A2010%3A348%3A0001%3A0016%3AEN%3APDF&usg=AOvVaw1hAsF3w4qZ2KU8EDDVvile
- EudraLex - Volume 1 - Pharmaceutical legislation for medicinal products for human use. Available online: https://ec.europa.eu/health/documents/eudralex/vol-1_en
- EudraLex - Volume 2 - Pharmaceutical legislation on notice to applicants and regulatory guidelines for medicinal products for human use. Available online: https://ec.europa.eu/health/documents/eudralex/vol-2_en
- Council Directive 89/381/EEC of 14 June 1989 extending the scope of Directives 65/65/EEC and 75/319/EEC on the approximation of provisions laid down by Law, Regulation or Administrative Action relating to proprietary medicinal products and laying down special provisions for medicinal products derived from human blood or human plasma. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX:31989L0381
- EudraLex - Volume 3 - Scientific guidelines for medicinal products for human use. Available online: https://ec.europa.eu/health/documents/eudralex/vol-3_en
- Regulation (EC) no 141/2000 of the European Parliament and of the council of 16 December 1999 on orphan medicinal products. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2000:018:0001:0005:en:PDF
- Community register of orphan medicinal products for human use. Available online: http://ec.europa.eu/health/documents/community-register/html/alforphreg.htm
- Plasma humanum ad separationem Ph. Eur. Monograph (0853) corrected. Eur. Pharmacopeia: Available online: http://online6.edqm.eu/ep902/; https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-9th-edition
- Human anti- D immunoglobulin for intravenous administration Ph. Eur. Monograph (1527). Eur. Pharmacopeia: Available online: http://online6.edqm.eu/ep902/; https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-9th-edition
- Human coagulation factor VIII Ph. Eur. Monograph (0275) and human coagulation factor VIII (rDNA) Ph. Eur. Monograph (1643). Eur. Pharmacopeia: Available online: http://online6.edqm.eu/ep902/; https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-9th-edition
- EudraLex - Volume 3 - Scientific guidelines for medicinal products for human use. Available online: https://ec.europa.eu/health/documents/eudralex/vol-3_en
- CPMP Note for guidance on the design, contribution and interpretation of studies validating the inactivation and removal of viruses. CPMP/BWP/268/95. Available online: http://www.ema.europa.eu/ema/268/95validating the inactivation and removal of viruses
- Guideline on the warning on transmissible agents in summary of product characteristics (SmPCs) and package leaflets for plasma-derived medicinal products. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=2ahUKEwiblJbLyZ7dAhWVwAIHHZ6tAjoQFjAAegQIABAC&url=http%3A%2F%2Fwww.ema.europa.eu%2Fdocs%2Fen_GB%2Fdocument_library%2FScientific_guideline%2F2011%2F12%2FWC500119001.pdf&usg=AOvVaw3eyf_xT0fmerd4BIzXS-26
- Virus safety evaluation of biotechnological investigational medicinal products. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000952.jsp&mid=WC0b01ac058002956c
- CHMP. Reflection paper on viral safety of plasma-derived medicinal products with respect to Hepatitis E virus. EMA/CHMP/BWP/723009/2014. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/06/WC500209354.pdf
- CPMP. Position Statement on West Nile Virus and Plasma Derived Medicinal Products. CPMP/BWP/3752/03. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Position_statement/2009/09/WC500003789.pdf
- Zaman SM, Hill FG, Palmer B, et al. The risk of variant Creutzfeldt-Jakob disease among UK patients with bleeding disorders, known to have received potentially contaminated plasma products. Haemophilia 2011;17:931-7. [Crossref] [PubMed]
- UK Department of Health: Blood-borne transmission of vCJD re-examination of scenarios. Available online: https://www.gov.uk/government/publications/blood-borne-transmission-of-vcjd-re-examination-of-scenarios
- Analyse du risque de transmission de la variante de la Maladie de Creutzfeldt-Jakob (vMCJ) par les produits de santé d’origine humaine. Available online: https://ansm.sante.fr/var/ansm_site/storage/original/application/31d59f6c76604b48cfbfc1a332a8ee64.pdf
- Position Paper on Creutzfeldt-Jakob disease and plasma-derived and urine-derived medicinal products. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000894.jsp&mid=WC0b01ac058002956b
- Investigation of manufacturing processes for PDMPs with regard to variant Creutzfeldt-Jakob disease risk. Available online: Investigation of manufacturing processes for PDMPs with regard to variant Creutzfeldt-Jakob disease risk
- Minimising the risk of transmitting animal spongiform encephalopathy agents via human and veterinary medicinal products. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000942.jsp&mid=WC0b01ac058002956c
- Questions and answers on bovine spongiform encephalopathies (BSE) and vaccines. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000945.jsp&mid=WC0b01ac058002956c
- Evaluation of bovine spongiform encephalopathies (BSE) - risk via the use of materials of bovine origin in or during the manufacture of vaccines. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_001650.jsp&mid=WC0b01ac058002956c
- First cases of BSE in USA and Canada: risk assessment of ruminant materials originating from USA and Canada. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000941.jsp&mid=WC0b01ac058002956c
- Guideline on the scientific data requirements for a plasma master File (PMF) Revision 1. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003663.pdf
-
Guideline on Requirements for Plasma Master File (PMF) Certification - Guideline on epidemiological data on blood transmissible infections. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/02/WC500202385.pdf
- Guideline on validation of immunoassay for the detection of Antibody to human immunodeficiency virus (anti-HIV) in plasma pools. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003685.pdf
- Validation of immunoassay for the detection of hepatitis B virus surface antigen in plasma pools. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000901.jsp&mid=WC0b01ac058002956b
- Requirements for plasma master file certification. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003747.pdf
- EMA Clinical investigation and Core SmPC guidelines for PDMPs. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000388.jsp&mid=WC0b01ac0580032ec8
- Clinical efficacy and safety: blood products (including biotech alternatives). Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000388.jsp&mid=WC0b01ac0580032ec8
- Regulation (EC) n) 1902/2006 amending paediatric regulation in which changes to the original text were introduced relating to decision procedures for the European Commission. Available online: https://ec.europa.eu/health//sites/health/files/files/eudralex/vol-1/reg_2006_1902/reg_2006_1902_en.pdf
- Draft guideline on clinical investigation of recombinant and human plasma-derived factor VIII products. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2017/10/WC500237866.pdf
- Peyvandi F, Mannucci PM, Garagiola I, et al. A Randomized Trial of Factor VIII and Neutralizing Antibodies in Hemophilia A. N Engl J Med 2016;374:2054-64. [Crossref] [PubMed]
- Van der Bom JG, Gouw SC, Rosendaal FR. Second-generation recombinant factor VIII and inhibitor risk: interpretation of RODIN study findings and implications for patients with haemophilia A. Haemophilia 2014;20:e171-4. [Crossref] [PubMed]
- Calvez T, Chambost H, d'Oiron R, et al. Analyses of the FranceCoag cohort support immunogenicity differences among one plasma-derived and two recombinant factor VIII brands in boys with severe haemophilia. A. Haematologica 2018;103:179-89. [Crossref] [PubMed]
- Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 8-11 January 2018. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2018/01/news_detail_002881.jsp&mid=WC0b01ac058004d5c1
- Batch Release for Human Biologicals: Vaccines, blood and plasma derivatives. Available online: https://www.edqm.eu/en/batch-release-human-biologicals-vaccines-blood-and-plasma-derivatives
- Control Authority of Batch release Vaccines and Blood products. Available online: https://ec.europa.eu/health/sites/health/files/files/pharmacos/news/gl981001_en.pdf
-
Human OCABR Guidelines - EDQM - Plasma humanum ad separationem European Pharmacopoeia monograph 5.0 01/2005: 0853 corrected. Eur. Pharmacopeia: Available online: http://online6.edqm.eu/ep902/; https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-9th-edition
- Human anti-D immunoglobulin for intravenous administration European Pharmacopoeia monograph 1527 (≤ 104 UI/ml). Eur. Pharmacopeia: Available online: http://online6.edqm.eu/ep902/; https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-9th-edition
- Report Blood, tissues, cells and organs stakeholder meeting Sept 2017. Available online: https://ec.europa.eu/health/blood_tissues_organs/policy/evaluation_en
- Transfusion and transplantation: Protection and Selection of donors project. Available online: Transfusion and transplantation: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=2ahUKEwiFw_H0mIbdAhUQz4UKHZ7KBbkQFjAAegQIABAB&url=https%3A%2F%2Fwww.transposeproject.eu%2F&usg=AOvVaw0eaK8N7OHpoAo4MX45gqPU
Cite this article as: Rossi F. The organization of transfusion and fractionation in France and its regulation. Ann Blood 2018;3:37.