
Role of the mini-pool cryoprecipitate technology for cost-saving and guarantee of local Factor VIII, Von Willebrand Factor and Fibrinogen product supply: Egypt experience
Introduction
Hemophilia A is a rare hereditary bleeding disorder that has a uniform global prevalence of one per ten thousand population (1). While the diagnosed and registered patients are very close to this estimated prevalence in developed countries, in developing and under developed countries the majority of patients are still not diagnosed (2). Treatment of hemophilia A relies on knowledgeable health care professionals, safe treatment products (CFCs), multidisciplinary hemophilia care centers and presence of strong national patient organization that can advocate for patients’ rights to have access to proper care wherever they are (3). In developed countries there is surplus of safe treatment products. In these countries the main challenge is the development of inhibitors to CFCs, where it is estimated that the incidence of inhibitor development in previously untreated patients (PUPs) with severe hemophilia A is more than 30% (4), especially when receiving recombinant FVIII products compared to plasma-derived preparations also containing von Willebrand factor (5). On the other hand, patients in developing countries have restricted or no access to CFCs (2). Fresh blood components such as fresh frozen plasma (FFP) and cryoprecipitate may be the main available treatment products. This exposes patients to complications of blood transfusion such as viral transfusion transmitted diseases and immunological complications (6). In the meantime limited product access results in frequent bleeding into musculoskeletal system, which results in severe pain and various degrees of handicapping (7). In addition lack of access to comprehensive treatment centers further complicates the situation as patients will not have proper management of their condition. Absence of strong patient organization to support their case leads to ignorance of the community about problems of patients with hemophilia and consequently lack of support from health authorities.
Inherited bleeding disorders (IBDs) in Egypt
Uniform hemophilia care started in Egypt 1971 by establishment of a unit for diagnosis of IBDs at Central Health Laboratories, a Hemophilia Treatment Center (HTC) at the Diabetes Institute as well as patient organization. Egyptian Society of Hemophilia (ESH) joined World Federation of Hemophilia (WFH) in 1971. ESH holds national registry for patients with IBDs. According to WFH 2015 annual global survey, the current registry of Egyptian IBDs includes 4,358 patients with hemophilia A, 1,062 patient with hemophilia B, 528 patient with von Willebrand Disease (VWD) as well as 1,168 patients with other IBDs (2). During the seventies, Egypt Vaccine and Serum Institute (Vac Sera) established a small fractionation facility that produced (non-virally inactivated) lyophilized cryoglobulin as well as lyophilized plasma from small pools of cryoprecipitate and plasma respectively. These products were used to treat patients with IBDs. Due to high prevalence of HCV infection among Egyptian population, and before the introduction of serological screening in the early nineties of the last century, many patients with IBDs became infected by contaminated fresh plasma components as well as lyophilized products from Vac Sera. In the late nineties, Vac Sera decided to close its small fractionation facility due to lack of viral safety of its products. This situation led to major shortage in treatment products for patients with hemophilia and other IBDs. As well, there was a general concern about the safety of plasma derived CFCs due to the epidemic of HIV infection during the seventies and eighties among patients with hemophilia in developed world (8,9). Recombinant CFCs were unaffordable to developing countries due to their high cost. Moreover, the high rate of development of FVIII inhibitors limited its procurement and use in Egypt (4,10), especially as the specialized knowledge, infrastructure, and products to treat such patients were, and still are, lacking.
Development of mini-pool fractionation technology
With all these challenges in mind a group of researchers from Egypt and France worked on the idea of developing an affordable and simple technology that can help developing countries to use domestic plasma for production of safe cryoprecipitate and plasma that can be used to treat patients with IBDs. The work of this group focused on the adaptation of solvent and detergent (S/D) virus inactivation technology used in the plasma fractionation industry to be used in blood establishments and national blood service centers. The work focused on development of single use sterile medical devices for S/D virus inactivation of mini-pools of cryoprecipitate and plasma (11,12). The development was industrialized in the form of two CE marked medical devices for S/D virus inactivation and 0.2 μm filtration for mini-pools of cryoprecipitate and plasma. The two devices were extensively validated for virus inactivation capacity, protein recovery, residual levels of TnBP (tri-n-buthyl-phosphate, a solvent) and Triton X45 (a detergent) as well as animal safety studies (13).
Pharmacokinetic study of S/D-F cryoprecipitate FVIII
Eighteen cryoprecipitate mini-pools, each made of 30 units of low volume, concentrated cryoprecipitate, were treated by solvent-detergent and 0.2 µm filtration (S/D-F) in a single-use CE-marked bag system. The S/D-F cryoprecipitate contained a mean of 10.5 IU/mL Factor VIII (FVIII), 17 mg/mL clottable fibrinogen, and >10 IU/mL von Willebrand Ristocetin co-factor activity. Anti-A and anti-B isoagglutinins were undetectable. The products have been infused in 11 severe (FVIII <1%) haemophilia A patients (mean age: 17.4 years; mean weight: 57.6 kg) at a dose close to 40 IU/kg. Patients were hospitalized for at least 36 h to determine FVIII recovery, half-life and clearance. They were also closely monitored for possible adverse events. None of the infused patients demonstrated reactions or adverse events even though they did not receive anti-allergic drugs or corticosteroids prior to infusion. The mean recovery of FVIII 10 min post-infusion was 69.7%. Mean FVIII half-life was 14.2 h and clearance was 2.6 mL/hr/kg. All patients had a bleeding free interval of 8–10 days post S/D-F cryoprecipitate infusion. The data show that S/D-F cryoprecipitate FVIII exhibits a normal pharmacokinetics profile, and support that it could be safely used for the control of acute and chronic bleeding episodes in hemophilia A patients (14).
Preparation of S/D treated cryoprecipitate
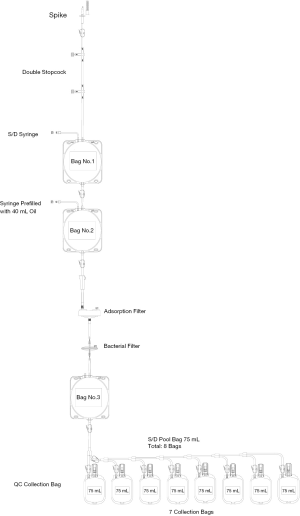
S/D virus inactivation medical device consists of a cascade of interconnected bags shown in Figure 1. The first two, 750 mL-oval bags, are used for two stages of S/D virus inactivation, followed by TnBP and Triton X 45 extraction by soybean oil. Interconnected to the two oval bags are two filters, one composed of charcoal and the other is a standard sterilization 0.2 µm filter for removal of residual S/D and clarification and purification of processed cryoprecipitate, respectively. Subsequent to 0.2 µm filtration, there is a third oval bag in which filtered cryoprecipitate is recovered. The latter is linked to a quality control bag as well as to 7 small bags for dispensing defined volumes of S/D cryoprecipitate in each one. By determining the concentration of FVIII, FI (clottable fibrinogen) and VWF per ml, it is possible to accurately dose and label each of the small storage bags (13) (Figure 2) Good manufacturing practice of cryoprecipitate production is described in the EDQM/Council of Europe “Guide to the preparation, use and quality assurance of blood components” as well as in the AABB technical manual (15,16). The cryoprecipitate used as starting material is almost depleted of plasma (“dry” or low-volume cryoprecipitate) and thereby concentrated. Under a laminar flow cabinet, mini-pools of “dry” cryoprecipitate (35 units) are individually and aseptically suspended in 5 mL of 5% saline glucose (licensed infusion solution obtained from local pharmaceutical industry) inside their blood bags. The suspended units are then pooled aseptically inside the first virus inactivation bag of S/D cryoprecipitate virus inactivation medical device. S/D virus inactivation is then performed in two stages according to the medical device user guide and essentially as previously described (13). The process time of this S/D cryoprecipitate mini-pool is on average 4 hours with technician hands-on of less than eighty minutes. The process can be easily adapted to blood establishments or national blood service centers which largely reduces the infrastructure cost compared to the fractionation industry. Thirty-five units of cryoprecipitate produced from quality apheresis source plasma or plasma recovered from whole blood and treated by the S/D medical device described above has an average FVIII yield of 4,000 IU. This means that the average FVIII recovery per liter of FFP is more than 500 IU which is much higher than the yield of less than 200 IU FVIII achieved during large-scale plasma fractionation (17). Thus, this device optimizes FVIII extraction and consequently avoids waste of natural resources. According to the price of the medical device to the end user and the yield of FVIII per device, the cost of one unit of FVIII is around 7 US cents. The produced S/D cryoprecipitate units are stored in a plasma freezer at −25 °C or colder for one year according to Egypt blood bank regulations. Since S/D cryoprecipitate is now shipped to hemophilia A treatment centers in Egypt as well as for home therapy, the product is transferred frozen at −20 °C or colder using mobile freezers or dry ice tanks. When used for home therapy, the recommendation is to keep it in a domestic or hospital freezer for less than 1 month.

Clinical experience with S/D cryoprecipitate
Twelve severe haemophilia A kids without FVIII inhibitor and negative for HBV, HCV and HIV serological markers were put on regular prophylaxis with the minipool S/D cryoprecipitate with a mean annual FVIII dose of 1,029 IU/kg (range, 545–1,684 IU/kg) were clinically followed for a period ranging from 2 to 5 years. Kids were regularly infused with S/D cryoprecipitate FVIII at a dose of 20 IU/kg body weight (BW) weekly. If a child developed breakthrough bleed, the same dose was increased to twice weekly and if more breakthrough bleeds happened, the same dose was given three times weekly. Follow-up of these kids showed that the mean annual bleeding rate was 2 as compared to one or more bleeds per month in kids who were not on prophylaxis. Quite importantly, no FVIII inhibitor development and no transmission of HIV, HCV or HBV were observed according to our regular check for inhibitors and markers for HCV, HBV and HIV infections every 6 months. No adverse events were reported during a mean of 41 months follow-up period (range, 24–51 months). The quality of life of these kids was found to be comparable to their normal age matched peers (18). Since 2013 more than 96,000 doses of S/D Cryoprecipitate FVIII (250 & 500 IU FVIII) were infused to more than 2,000 patients with hemophilia A (PWH), VWD, FI and FXIII deficiency in more than 12 hemophilia treatment centers in Egypt. Efficacy and safety were comparable to plasma derived and recombinant CFCs. There were no reports on severe or moderate adverse events. Four patients with FVIII inhibitors were found in a cohort of 70 previously transfused patients (6% prevalence) who received mini-pool S/D cryoprecipitate among other blood components and industrial FVIII derivatives. However, it is not known if the inhibitor development was related to S/D cryoprecipitate as other products were used alternatively. From these relatively limited data it looks that S/D cryoprecipitate FVIII has low immunogenicity compared to standard CFCs. It is worth mentioning that longer surveillance for development of FVIII inhibitors in previously untransfused patients when treated by S/D cryoprecipitate FVIII to confirm that it is less immunogenic compared to plasma derived and recombinant CFCs. S/D virus inactivation technology only inactivates lipid enveloped viruses. Therefore, there might be a small risk of transmission of lipid non enveloped viruses such as hepatitis A and Parvo B19. The small size of the pool with very low residual plasma volume may largely limit this risk compared to large pools of plasma fractionation. Hepatitis A vaccination of patients receiving S/D cryoprecipitate may be advised to further reduce this risk.
S/D cryoprecipitate was also used in surgical prophylaxis for PWH, VWD and FI deficiency. The surgical procedures ranged from minor, like tooth extraction, circumcision, to major such as abdominal exploration and hip arthroplasty. Surgical prophylaxis protocols were according to WFH Guidelines on management of hemophilia for limited resource settings. The product showed efficacy in preventing bleeding during and after surgery, and there were no reported cases of adverse events.
Egypt experience—economic implications
Based on these results, it is estimated that the mean annual cost of S/D cryoprecipitate FVIII prophylaxis is 72 USD/kg BW/year. Taking into account that the cost of plasma derived CFCs in Egypt is on average 21 US cents per unit of FVIII, the mean annual cost of prophylaxis is much higher (216 USD/kg/year) corresponding to 3 times the cost of this S/D cryoprecipitate FVIII. An additional important economic feature is the very low incidence of development of inhibitors (6%) compared to a range from 20% to 35.4% when using plasma derived or recombinant FVIII CFCs (4,5). Severe hemophilia A patients with inhibitors will need very expensive by- passing agents to control bleeding episodes. They will also need immune tolerance induction (ITI) to eradicate the inhibitors (19). The cost of ITI is prohibitively expensive to developing countries. It is also a very complex treatment modality that needs very advanced hemophilia treatment centers which do not exist in most of developing countries. According to different guidelines for ITI, the minimum tolerance protocol is infusion of FVIII at a dose of 25–50 IU/kg three times weekly for periods that may range from 6 to 33 months (19). To induce immune tolerance in a patient with FVIII inhibitors there will be a need for infusion of 4,000–8,000 IU FVIII/kg BW annually for a minimum of 6 months to almost 3 years. Therefore, the use of S/D cryoprecipitate is an extremely pragmatic and efficient option that largely reduces risks of inhibitor development, thereby decreasing morbidity and cost of treatment. For the same reasons, it is wise to avoid or limit use of recombinant products for IBD treatment in countries without appropriate economics and/or infrastructure at least for the first 50 FVIII exposure days in PUPs. Another important feature of S/D cryoprecipitate is its richness in VWF, Fibrinogen and FXIII (13,20). This implies that, as our clinical experience is evidencing, it can be efficiently used to treat patients with VWD, Fibrinogen and FXIII deficiency. Industrial VWF, Fibrinogen and FXIII concentrates are both expensive and of limited availability, which make them rarely or not at all accessible to patients living in developing countries. The price of one international unit of plasma derived or recombinant FVIII may range between 20 and 42 US cents. In Egypt, the lowest price of one international unit of plasma derived FVIII is 21 US cents while the cost of processing of S/D cryoprecipitate is 7 US cents per one international unit FVIII. It is worthwhile to point out that in the interval of 4 years, the feasibility rate of FVIII production accounted for 8,150 medical devices of S/D treated cryoprecipitate. This allowed to process 285,250 units of cryoprecipitate providing 53,631 bags each with a dose of 250 IU FVIII and 43,895 bags each with a dose of 500 IU FVIII.
The total number of S/D cryoprecipitate FVIII produced during this period was around 32 million international units with a cost of USD 2.45 million. Importing similar quantity of FVIII would have cost about USD 7 million. Therefore, S/D cryoprecipitate FVIII allowed a substantial saving in Egypt of about USD 4.5 million.
Conclusions
Patients with IBDs living in developing countries do not have access to safe treatment products yet, and certainly for many years to come. Although WFH has for long adopted the vision of “Treatment for All”, which is largely based on channeling donations as well as encouraging wealthy governments to procure CFCs, the situation did not much improve in developing countries. Thus, there is a need to have additional strategies at local and national levels to improve the situation. WHO encourages governments worldwide to enhance safety and availability of blood as an essential medicine. Preparation and processing of cryoprecipitate by S/D virus inactivation method from domestic plasma resources can largely increase availability of safe treatment products for patients with IBDs at an affordable cost and with better guarantee of supply that is not totally dependent on donations or imports. Egypt experience in the production and use of S/D cryoprecipitate demonstrate efficacy, safety and economic viability of such approach. Implementation of the technology in other developing countries can be a means to improve care for patients with IBDs to achieve the vision of WFH: “Treatment for All”. We are now developing a similar approach for the production of mini-pool immunoglobulins (21). The results of clinical studies are showing that this mini-pool immunoglobulin preparation exhibits good tolerance and safety, as well as effectiveness in the treatment of immune thrombocytopenia (22). Mini-pool immunoglobulins prepared from local plasma in a process comparable with the production of mini-pool cryoprecipitate therefore offers further perspective for local supply of plasma derived products.
Acknowledgments
Authors would like to acknowledge Egyptian Company for Biological Sciences (ECBS) for providing the data related to the production of S/D cryoprecipitate. Special thanks to the staff of Shabrawishi Hospital hemophilia treatment center who provided data on their prophylaxis program for children with hemophilia A.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Blood for the series “Plasma Fractionation”. The article has undergone external peer review.
Conflicts of Interest: The series “Plasma Fractionation” was commissioned by the editorial office without any funding or sponsorship. Thierry Burnouf served as an unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Blood from Feb 2017 to Feb 2020. M El Ekiaby is a technology developer of S/D Medical Device, shareholder in the Viral Inactivated Plasma Systems SA (VIPS), Switzerland; producer of the medical device and director of Shabrawishi Hospital Blood Transfusion Center. T Burnouf is an inventor of patents assigned to VIPS. M Radosevich is also an inventor of patents assigned to VIPS. H Goubran is also an inventor of patents assigned to VIPS. T Burnouf, H Goubran, and M Radosevich are also the technology developers, and declare no financial interest. A El Ekiaby is a first degree relative to M El Ekiaby.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia 2013;19:e1-47. [Crossref] [PubMed]
- World Federation of Hemophilia. Annual Global Survey 2015. Available online: http://www.wfh.org, 2016.
- Berntorp E, Dargaud Y, Hart D, et al. The second Team Haemophilia Education Meeting, 2016, Frankfurt, Germany. Eur J Haematol 2017;98:1-15. [Crossref] [PubMed]
- Ettingshausen CE, Kreuz W. Recombinant vs. plasma-derived products, especially those with intact VWF, regarding inhibitor development. Haemophilia 2006;12:102-6. [Crossref] [PubMed]
- Peyvandi F, Mannucci PM, Garagiola I, et al. A Randomized Trial of Factor VIII and Neutralizing Antibodies in Hemophilia A. N Engl J Med 2016;374:2054-64. [Crossref] [PubMed]
- Abdelwahab MS. Reply: To PMID 22366833. Ann Saudi Med 2013;33:81-2.
- Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet 2016;388:187-97. [Crossref] [PubMed]
- del Amo J, Pérez-Hoyos S, Moreno A, et al. Trends in AIDS and mortality in HIV-infected subjects with hemophilia from 1985 to 2003: the competing risks for death between AIDS and liver disease. J Acquir Immune Defic Syndr 2006;41:624-31. [Crossref] [PubMed]
- Jewell NP. Quantifying the source of infection for HIV-infected hemophiliacs in the U.K. from 1979 to 1984. Stat Med 2009;28:1464-72. [Crossref] [PubMed]
- Franchini M, Coppola A, Rocino A, et al. Systematic review of the role of FVIII concentrates in inhibitor development in previously untreated patients with severe hemophilia a: a 2013 update. Semin Thromb Hemost 2013;39:752-66. [Crossref] [PubMed]
- Burnouf T, Goubran HA, Radosevich M, et al. A minipool process for solvent-detergent treatment of cryoprecipitate at blood centres using a disposable bag system. Vox Sang 2006;91:56-62. [Crossref] [PubMed]
- Burnouf T, Goubran HA, Radosevich M, et al. A process for solvent/detergent treatment of plasma for transfusion at blood centers that use a disposable-bag system. Transfusion 2006;46:2100-8. [Crossref] [PubMed]
- El-Ekiaby M, Sayed MA, Caron C, et al. Solvent-detergent filtered (S/D-F) fresh frozen plasma and cryoprecipitate minipools prepared in a newly designed integral disposable processing bag system. Transfus Med 2010;20:48-61. [Crossref] [PubMed]
- El-Ekiaby M, Goubran HA, Radosevich M, et al. Pharmacokinetic study of minipooled solvent/detergent-filtered cryoprecipitate factor VIII. Haemophilia 2011;17:e884-8. [PubMed]
- EDQM. Guide to the Preparation, Use and Quality Assurance of Blood Components. 18 ed, 2015.
- American Association of Blood Banks (AABB). Technical Manual. 17 ed. 2011.
- Burnouf T. Modern plasma fractionation. Transfus Med Rev 2007;21:101-17. [Crossref] [PubMed]
- Riddell A, Chuansumrit A, El-Ekiaby M, et al. Diagnostic laboratory for bleeding disorders ensures efficient management of haemorrhagic disorders. Haemophilia 2016;22:90-5. [Crossref] [PubMed]
- Valentino LA, Kempton CL, Kruse-Jarres R, et al. US Guidelines for immune tolerance induction in patients with haemophilia a and inhibitors. Haemophilia 2015;21:559-67. [Crossref] [PubMed]
- Burnouf T, Caron C, Radosevich M, et al. Properties of a concentrated minipool solvent-detergent treated cryoprecipitate processed in single-use bag systems. Haemophilia 2008;14:956-62. [Crossref] [PubMed]
- El-Ekiaby M, Vargas M, Sayed M, et al. Minipool caprylic acid fractionation of plasma using disposable equipment: a practical method to enhance immunoglobulin supply in developing countries. PLoS Negl Trop Dis 2015;9:e0003501 [Crossref] [PubMed]
- Elalfy M, Reda M, Elghamry I, et al. A randomized multicenter study: safety and efficacy of mini-pool intravenous immunoglobulin versus standard immunoglobulin in children aged 1-18 years with immune thrombocytopenia. Transfusion 2017;57:3019-25. [Crossref] [PubMed]
Cite this article as: El Ekiaby M, Burnouf T, Goubran H, Radosevich M, El Ekiaby A. Role of the mini-pool cryoprecipitate technology for cost-saving and guarantee of local Factor VIII, Von Willebrand Factor and Fibrinogen product supply: Egypt experience. Ann Blood 2018;3:22.


