
Building blocks of the viral safety margins of industrial plasma products
Background
Plasma-derivatives have been amongst the earliest successes of medicinal biotechnology, with human serum albumin (HSA) first developed by Cohn and coworkers under the impression of the medical needs that arose from the dramatic battlefield mortalities during World War II (1). In the 1950s, Bruton recognized the possibility of replacing the missing serum electrophoresis gamma globulin band in one of his patients suffering from recurring infectious diseases by substitution with gamma globulin from fractionated plasma, thus providing for an initial success in the treatment of immune deficiency (2). The 1960s saw Judith Graham Pool developing the cryo-precipitate fraction of human plasma which contains some concentrated clotting factors, including factor VIII, into an effective treatment for hemophilia (3). These early accomplishments were, however, overshadowed from the early 1980s by the recognition of virus transmission through these medically important plasma-derived products (4), as reviewed by Evatt, the CDC lead investigator at the time (5). While blood transfusions at the time also transmitted human immunodeficiency virus (HIV), the complications associated with plasma products were more impactful to the treated communities, as the pooling of thousands of plasma units for a single batch of product resulted in a significant amplification of transmission, potentially from single or few donors to a large number of recipients. As information about the causative agent became available, preventive measures such as the exclusion of individuals at higher risk of carrying HIV from the donor pool, testing initially for surrogate markers (hepatitis B core antigen) and later the virus itself (6), and finally virus inactivation by implementation of heat treatments for liquid or lyophilized factor concentrates, were rapidly instituted.
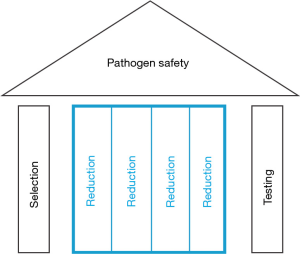
Today, plasma-derived products, through the implementation of this array of measures, now commonly referred to as the safety tripod (Figure 1), feature very substantial safety margins. And while the volatile microbiological environment of human plasma donors is understandably beyond perfect control, it is all the more important to recognize that the virus inactivation and removal processes, as embedded into current state-of-the-art manufacturing processes of plasma derivatives, are the quantitatively most important stronghold to avoid any unwelcome reminders of past exposures in the future. To substantiate this claim, the experimental and epidemiological evidence that supports the notion will be reviewed.
Selection
The first intervention that became available to reduce the likelihood of virus transmissions, while still keeping the medically so successful plasma-derived treatment options available to those in need, was the selection of healthy donors and the deferral of donors with known risk of carrying infectious disease agents. History may help to understand debates of today, as the virus-caused immune deficiency now called AIDS was initially termed gay-related immune deficiency (GRID) by the lay press (7), due to an overrepresentation of HIV infections in the community engaged in the respective sexual practices of higher risk for HIV transmission. The exclusion of these individuals at statistically elevated risk of carrying an infectious disease form blood and also plasma donation was thus a most logical choice. Similarly, other life style indicators, such as tattooing and piercing, which at unregulated parlors were associated with an increased risk of contracting an infectious disease, were and are still used to defer people from donation. The increasing regulatory oversight of application practices for tattoos and piercings has greatly reduced the risk of infectious complications now. In addition, these body styling actions have earlier symbolized identification with the margins of society, which for other reasons may have had an increased risk of contracting infectious diseases, whereas they are now common throughout many segments of society, including, students. With these developments the actual value of this deferral practice may have become questionable.
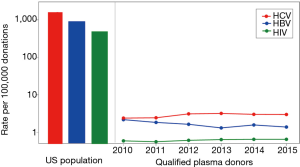
Still to date though, the selection of plasma donors of the desired safety profile is part of the quality management process around plasma collection. A comprehensive set of voluntary standards for plasma fractionation has been developed by the Plasma Products Therapeutics Association (PPTA), and is certified against within the International Quality Plasma Program (IQPP) (8). To illustrate one of the accomplishments, a comparison of the rates of HIV, hepatitis B virus (HBV) and hepatitis C virus (HCV) infections in the general US population, versus qualified plasma donors as defined by PPTA’s IQPP* is helpful. As can be seen (Figure 2), the US population rates of infection with these viruses are around 1,000 per 100,000, i.e., ~1%, yet the rates for qualified donors are approximately 1,000-fold lower. The IQPP quality management system thus generates an up to 1,000-fold or 3 log10 improvement of safety margins by selection of a donor pool of superior health status with respect to these infectious diseases.
Testing
The application of testing to plasma for fractionation has certainly improved safety margins of plasma derivatives, but was and is at the same time insufficient to, by itself, completely safeguard those products. The scientific reason explaining this situation is the difference between a “non-reactive” test result, i.e., the concentration of a marker for the presence of, or the infectious agent itself, is below the limit of detection of the respective assay, versus the complete absence of an infectious agent that cannot be ascertained by testing ever.
As best illustrated by the historical sequence of implementing different tests for infectious agents for units of blood collected for transfusion, which is as a good surrogate for the effect of similar testing for the safety of plasma collected for fractionation, the risk of using contaminated units has been significantly reduced over time. Quantitatively, these improvements in final product safety margins were in the range of ~100-fold for HBV, i.e., 2 log10, or 10,000-fold for HIV, i.e., 4 log10 (9).
This increase in safety margins as afforded by testing is, however, limited to infectious agents that are known to circulate in the donor community. And, as the blood and plasma products communities have come to witness over time, there has been a steady stream of newly discovered or newly emerging infectious agents that might enter the potential donor pool, often resulting in questions about the safety margins of the respective products. Inherently, all of these emerging challenges cannot be eliminated by testing before becoming potentially impactful for blood- or plasma-product recipients, and even after an exposure has been recognized, the development of adequately sensitive, yet affordable, test systems has taken and will still need time with current testing approaches.
For the safety margins of blood components for direct transfusion, the testing of donations combined with the selection of low-risk donors have been the only interventions traditionally used to enhance final product safety margins. This is fundamentally different to the safety margins of plasma derivatives which, beyond the contributions of donor selection and donation testing, enjoy the additional contributions of the particularly potent virus inactivation and removal processes embedded into their manufacturing processes: quantitatively, their contributions to final product safety margins are orders of magnitude more potent than selection and testing.
Reduction
Driven by the events of the early 1980s, new manufacturing processes were designed for plasma derived medicinal products, with the major goal to implement additional steps with the sole purpose of inactivation or removal of viruses, i.e., dedicated virus reduction steps. With the implementation of each of those steps, the virus safety margins of plasma derivatives were increased on a logarithmic scale.
Technically, the first approach taken was the use of heat for virus inactivation, either by pasteurization for liquid product formulations, or by dry heat or vapor heat for lyophilized product. Not much later, a group around Bernhard Horowitz developed what became one of the most effective ways to inactivate lipid-enveloped viruses, while maintaining the biological activity of essentially all plasma proteins, the solvent-detergent treatment (10). Mechanistically, it is assumed that treatment of a lipid-enveloped virus particle with various solvent-detergent combinations deprives the particle of its lipid envelope, and thus of recognition by the surface receptor proteins, which renders the virus non-infectious (11,12). Due to its powerful inactivation capacity for lipid-enveloped viruses, while preserving the biological function of medicinal products, solvent-detergent processes are now almost universally used, beyond plasma-derived products also for recombinant protein therapies that were developed much later.
In the 1990s then, nanofilters became available, i.e., filters with a particularly well-controlled pore size distribution in the nanometer (nm) range that allowed for the effective removal of virus particles, while passage of the therapeutic proteins was almost completely maintained. With filters of nominal pore sizes in the 35 nm range initially limited to the removal of viruses of somewhat larger size (13,14), or smaller viruses bound by antibodies which significantly increases their effective filtration size (15), filters with pore sizes in the 15–20 nm range are now widely available that allow for the effective removal of really any of the viruses of concern for human health, as well as potentially prions (16), the causative agents for the now practically extinct and originally man-made diseases bovine spongiform encephalopathy (BSE) and variant Creutzfeldt-Jacob disease (vCJD).
The implementation of these advancements in virus reduction technologies into the production processes of plasma derivatives was strongly encouraged by and quickly reflected in regulatory guidance around the viral safety of plasma products in general, particularly noteworthy the European guidelines on virus validation studies (17) and on plasma-derived medicinal products (18), which were both developed and issued in the first year of existence of the then new European regulatory body.
The quantitative differences in the contributions of donor selection, donation testing and virus reduction by dedicated manufacturing process steps to final product safety margins became apparent in great clarity with the emergence of West Nile virus (WNV) in the US in 1999, which also resulted in transmission of the virus through transfusion of blood components from 2002 and onwards, but did not challenge the safety margins of stable plasma-derivatives as manufactured at industrial scale.
The data collected from donor deferral and donation testing algorithms as applied to many millions of transfused blood donations (19) allowed for the calculation of the relative quantitative contributions of these interventions (20): the selection of (apparently) healthy donors has, based on the absence of any symptoms in approximately 80% of those carrying the virus in their blood, provided a rather marginal contribution to transfused blood component safety margins, that is, a reduction of risk by approximately 20% or 0.1 log. Application of what is still the most modern technology available for large-scale testing of blood donations, that is, nucleic acid testing (NAT), has reduced WNV transmissions through transfused blood components by approximately 90% or 1 log. For manufactured plasma derivatives, the inclusion of pathogen reduction steps, validated for WNV reduction after the first cases of blood transfusion transmission had been reported, demonstrated a risk reduction in the range of million-fold or higher per dedicated virus reduction step, that is, a reduction of risk by more than 6 log per step. And as expected from these significant quantitative differences, rare cases of transfusion-associated WNV infection still do occur (21,22), despite use of the most modern NAT and algorithms, yet plasma derivatives have maintained their pristine safety record, based on the significant virus inactivation and removal capacity of their manufacturing processes that was experimentally verified for WNV (23).
The history of emerging and re-emerging or translocated viruses, reflective of the volatile microbiological environment of humans as well as the massive streams of global travel and commerce that have literally made the world the “global village” as originally economists have called it, did, however, not stop with WNV. Since the turn of the millennium the safety margins of human-derived medicinal products have been challenged by epidemics of several of the generally more easily inactivated lipid-enveloped viruses, such as H5N1 influenza virus (H5N1), Chikungunya virus (CHIKV), and most recently Zika virus (ZIKV), but then also by typically more resistant non-lipid enveloped virus such as the hepatitis E virus (HEV). All of these episodes have generated an understandable level of unease and anxiety, and to address these legitimate concerns, as well as to support regulatory decision-making processes, the assumptions around products safety margins vis-à-vis these new challenges needed a critical appraisal. With large parts of the safety margins of plasma products based on the virus reduction capacity of their manufacturing processes, experimental verification of earlier conclusions was and is considered prudent, if not necessary.
With the emergence of BSE, a man-made infectious disease of cattle that was generated by making them feed on the proteinaceous remains of their likes, and the consequential transmission through the food chain which resulted in a new disease of humans, vCJD, the area of human-derived medicinal products experienced yet another, and in some ways unprecedented challenge: the potential for transmission of an infectious agents that, after research that resulted in two Noble prizes, for now, is understood to be the misfolded conformation of a protein only. With the recognition that indeed vCJD was transfusion-transmissible (24), the natural questions around the safety margins of plasma derivatives also had to be answered. And as in some ways expected, the manufacturing processes of plasma derivatives did what they were designed to do, i.e., remove a large number of different proteins that co-exist with any therapeutically interesting protein in plasma to purify the final medicinal product. This purification also resulted in a significant level of removal for the prion protein, as was experimentally verified by studies with various prion proteins (25), and further substantiated by collaborative efforts of the plasma products industry (26).
The results of several of these experimental verification studies which required newly established virus culture systems or even prion in vitro and in vivo infectivity assays, which could often only be conducted under significant biosafety containment conditions, and always under fairly dramatic time pressure to not repeat any mistakes of the past, have in part been made public, ultimately to provide for peace of mind for the plasma protein user communities; for reference, see examples on WNV (23), H5N1 (27), CHIKV (28), ZIKV (29-31), and HEV (32-34), as well as prion diseases (25,26).
Labile blood components for transfusion
Donor selection and donation testing have also been implemented into the system designed to provide for a safe supply with labile blood components for transfusion. Beyond these efforts though, we are witnessing the implementation of the contribution that has been so powerful in enhancing virus safety margins for plasma derivatives also for blood components, i.e., adoption of virus inactivation processes into their manufacturing schemes.
Specifically, the INTERCEPT™ (Cerus Corporation, USA), Mirasol® (Terumo BCT, USA) and THERAFLEX® UV (Macopharma, France) virus inactivation technology platforms are at various stages of development or licensure, or even available at the market for platelets and plasma; for a recent review see (35). As all these technologies have limitations with respect to the inactivation capacity against certain viruses, e.g., INTERCEPT could not prevent HEV transmission (36), Mirasol incompletely inactivated HIV and WNV (37), and THERAFLEX had a rather modest level of effectiveness against HIV (38), their use will not make the currently employed virus testing regimes obsolete. However, an algorithm that would allow for testing of blood donations in a cost-effective mini-pool scheme rather than the currently utilized single unit practice to identify and then to remove and discard the highly loaded units, combined with a virus inactivation technology that could handle the lower residual virus loads, is conceptually attractive. It is worth mentioning that with the described approach the savings generated by reduced numbers of virus tests might—at least in part—offset the additional cost for virus inactivation. Combined with the results of initiatives that are in the process of refining guidelines for the administration of optimal amounts of blood products in hemotherapy (39) which have already reduced the volume of transfused units, the new approach towards transfusion safety here described may not dramatically change the overall system cost while embedding better safety margins against existing and potentially emerging virus concerns. In addition, the currently available inactivation technologies have all shown significant potential in eliminating infectious bacteria, spirochetes and parasites (35).
Biotechnology, and advanced therapy medicinal products (ATMPs)
As the measures taken to eliminate virus concerns around the use of plasma derivatives have been particularly successful, a conceptually very similar approach has been taken to enhance the safety margins of biotechnology products, and the principle has been embedded into applicable guidance documents (40): three principal, complementary approaches have evolved to control the potential viral contamination of biotechnology products: (I) selecting and testing cell lines and other raw materials, including media components, for the absence of undesirable viruses which may be infectious and/or pathogenic for humans; (II) assessing the capacity of the production processes to clear infectious viruses (= reduction); (III) testing the product at appropriate steps of production for absence of contaminating infectious viruses.
The line of thought is another description of the safety tripod (Figure 1).
Equally, for ATMPs, the same safety tripod principles shall be applied, i.e., product safety as a result of the contribution of selection, testing and reduction. With our industry’s proclivity for innovation and sometimes forgotten lessons of the past [Charlene Banard (41)], however, it is of unchanged importance now to keep those learnings from the past fresh in our minds, and to utilize the concepts developed for mitigation of earlier concerns, to avoid future complications: those who cannot remember the past are condemned to repeat it (42).
And already, the first unfortunate complication has been seen, quite unfortunately, with the biological manufacturing platform of the first licensed gene therapy product found contaminated with an adventitious virus (43), despite all the compendial cell bank testing necessary for a successful license application. It may be noted that the contaminant was an insect virus unlikely to be infectious for human recipients of the treatment, but a likelihood argument is clearly not the appropriate approach towards the biological safety of medical products.
Where the active ingredient for some of these innovative products may itself be a virus, or even whole cells in the case of cell-based therapies and tissue engineering applications, arguably the implementation of virus reduction steps as traditionally designed into the downstream manufacturing process may be difficult, if not impossible. More innovatively, these interventions will likely have to move into the upstream, to form a virus barrier there, ideally followed by a functionally closed downstream manufacturing process to avoid any contamination there. For the technical embodiment, however, the utility of high-temperature short time (HTST), ultraviolet irradiation (UV) as well as nanofiltration has been demonstrated already, with nanofiltration probably the least process invasive and most universally usable approach. In support of the approach, an increasing number of virus filters specifically designed for the needs of upstream applications are now becoming available, i.e., filters that can tolerate even larger volumes at affordable cost.
Conclusions and outlook
Driven by the challenges associated with the transmission of infectious disease through industrial plasma products, different pathogen safety measures have been deployed and proven to be effective in safeguarding these important medicines. Conceptually, these interventions can be summarized in the safety tripod, i.e., the combination of donor selection, donation testing and finally, and quantitatively most importantly, virus inactivation and removal processes as embedded into their manufacturing processes. The virome of human plasma for fractionation has now been tested and analyzed by modern metagenomics/molecular biology methods, and has not revealed any surprises (44), so that plasma derivatives currently on the market enjoy substantial safety margins.
Still, the microbiological environment of human remains volatile, and thus continuous vigilance is a prime responsibility for those in charge of pathogen safety for any biological medicinal product. And with every emerging concern the reassessment and potentially verification of assumptions around product safety margins is prudent.
Due to the proven value of the safety tripod, the concept has been used to keep biotechnology products safe, and currently we witness the application of the very same principles to ATMPs.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Thierry Burnouf) for the series “Plasma Fractionation” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2018.02.01). The series “Plasma Fractionation” was commissioned by the editorial office without any funding or sponsorship. The author is employed by Baxter AG, now part of Shire, and has stock interest in Shire, a manufacturer of plasma derivatives, biotechnology products and gene therapies.
Ethical Statement: The author is accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
*Potential donors must pass two separate medical screenings and testing for HIV, HBV and HCV on two different occasions. Only after satisfactory screenings and negative test results does that person become a qualified donor. If a donor does not return within 6 months, that person loses his/her qualified donor status and must qualify again. This standard means that plasma from a one-time-only donor (even when all test results are negative) cannot be used for further manufacture. The standard results in committed donors and eliminates the risk that so-called “test-seekers” are accepted. Available online: http://www.pptaglobal.org/safety-quality/standards/iqpp; accessed October 13, 2017
References
- Cohn EJ, Oncley JL, Strong LE, et al. Chemical, Clinical, and Immunological Studies on the Products of Human Plasma Fractionation. I. The Characterization of the Protein Freactions of Human Plasma. J Clin Invest 1944;23:417-32. [Crossref] [PubMed]
- Bruton OC. Agammaglobulinemia. Pediatrics 1952;9:722-8. [PubMed]
- Pool JG, Gershgold EJ, Pappenhagen AR. High-Potency Antihaemophilic Factor Concentrate Prepared from Cryoglobulin Precipitate. Nature 1964;203:312. [Crossref] [PubMed]
- Centers for Disease Control (CDC). Pneumocystis carinii pneumonia among persons with hemophilia A. MMWR Morb Mortal Wkly Rep 1982;31:365-7. [PubMed]
- Evatt BL. The tragic history of AIDS in the hemophilia population, 1982-1984. J Thromb Haemost 2006;4:2295-301. [Crossref] [PubMed]
- McDougal JS, Cort SP, Kennedy MS, et al. Immunoassay for the detection and quantitation of infectious human retrovirus, lymphadenopathy-associated virus (LAV). J Immunol Methods 1985;76:171-83. [Crossref] [PubMed]
- Altman LK. New Homosexual Disorder Worries Health Officials. Available online: http://www.nytimes.com/1982/05/11/science/new-homosexual-disorder-worries-health-officials.html?pagewanted=all
- PPTA. International Quality Plasma Program (IQPP). Available online: http://www.pptaglobal.org/safety-quality/standards/iqpp
- Busch MP, Kleinman SH, Nemo GJ. Current and emerging infectious risks of blood transfusions. JAMA 2003;289:959-62. [Crossref] [PubMed]
- Horowitz B, Wiebe ME, Lippin A, et al. Inactivation of viruses in labile blood derivatives. I. Disruption of lipid-enveloped viruses by tri(n-butyl)phosphate detergent combinations. Transfusion 1985;25:516-22. [Crossref] [PubMed]
- Simons K, Helenius A, Garoff H. Solubilization of the membrane proteins from Semliki Forest virus with Triton X100. J Mol Biol 1973;80:119-33. [Crossref] [PubMed]
- Helenius A, Soderlund H. Stepwise dissociation of the Semliki Forest Virus membrane with trition X-100. Biochim Biophys Acta 1973;307:287-300. [Crossref] [PubMed]
- Roberts PL. Value of virus filtration as a method for improving the safety of plasma products. Vox Sang 1995;69:82-3. [Crossref] [PubMed]
- Burnouf T. Value of virus filtration as a method for improving the safety of plasma products. Vox Sang 1996;70:235-6. [Crossref] [PubMed]
- Kreil TR, Wieser A, Berting A, et al. Removal of small nonenveloped viruses by antibody-enhanced nanofiltration during the manufacture of plasma derivatives. Transfusion 2006;46:1143-51. [Crossref] [PubMed]
- Tateishi J, Kitamoto T, Mohri S, et al. Scrapie removal using Planova virus removal filters. Biologicals 2001;29:17-25. [Crossref] [PubMed]
- Note for Guidance on Virus Validation Studies: The Design, Contribution and Interpretation of Studies Validating the Inactivation and Removal of Viruses. 1996. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003684.pdf
- Guideline on plasma-derived medicinal products. 2011. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/07/WC500109627.pdf
- Petersen LR, Epstein JS. Problem solved? West Nile virus and transfusion safety. N Engl J Med 2005;353:516-7. [Crossref] [PubMed]
- Farrugia A, Kreil TR. Reflections on the emergence of chikungunya virus in the United States: time to revisit a successful paradigm for the safety of blood-derived therapies. Transfusion 2015;55:224-6. [Crossref] [PubMed]
- Montgomery SP, Brown JA, Kuehnert M, et al. Transfusion-associated transmission of West Nile virus, United States 2003 through 2005. Transfusion 2006;46:2038-46. [Crossref] [PubMed]
- Groves JA, Shafi H, Nomura JH, et al. A probable case of West Nile virus transfusion transmission. Transfusion 2017;57:850-6. [Crossref] [PubMed]
- Kreil TR, Berting A, Kistner O, et al. West Nile virus and the safety of plasma derivatives: verification of high safety margins, and the validity of predictions based on model virus data. Transfusion 2003;43:1023-8. [Crossref] [PubMed]
- Urwin PJ, Mackenzie JM, Llewelyn CA, et al. Creutzfeldt-Jakob disease and blood transfusion: updated results of the UK Transfusion Medicine Epidemiology Review Study. Vox Sang 2016;110:310-6. [Crossref] [PubMed]
- Lee DC, Stenland CJ, Miller JL, et al. A direct relationship between the partitioning of the pathogenic prion protein and transmissible spongiform encephalopathy infectivity during the purification of plasma proteins. Transfusion 2001;41:449-55. [Crossref] [PubMed]
- Cai K, Groner A, Dichtelmuller H, et al. Prion removal capacity of plasma protein manufacturing processes: A data collection from PPTA member companies. Transfusion 2013;53:1894-905. [Crossref] [PubMed]
- Kreil TR, Unger U, Orth SM, et al. H5N1 influenza virus and the safety of plasma products. Transfusion 2007;47:452-9. [Crossref] [PubMed]
- Leydold SM, Farcet MR, Kindermann J, et al. Chikungunya virus and the safety of plasma products. Transfusion 2012;52:2122-30. [Crossref] [PubMed]
- Blümel J, Musso D, Teitz S, et al. Inactivation and removal of Zika virus during manufacture of plasma-derived medicinal products. Transfusion 2017;57:790-6. [Crossref] [PubMed]
- Roth NJ, Schafer W, Popp B, et al. Verification of effective Zika virus reduction by production steps used in the manufacture of plasma-derived medicinal products. Transfusion 2017;57:720-1. [Crossref] [PubMed]
- Farcet MR, Kreil TR. Zika virus is not thermostable: very effective virus inactivation during heat treatment (pasteurization) of human serum albumin. Transfusion 2017;57:797-801. [Crossref] [PubMed]
- Yunoki M, Yamamoto S, Tanaka H, et al. Extent of hepatitis E virus elimination is affected by stabilizers present in plasma products and pore size of nanofilters. Vox Sang 2008;95:94-100. [Crossref] [PubMed]
- Yunoki M, Tanaka H, Takahashi K, et al. Hepatitis E virus derived from different sources exhibits different behaviour in virus inactivation and/or removal studies with plasma derivatives. Biologicals 2016;44:403-11. [Crossref] [PubMed]
- Farcet MR, Lackner C, Antoine G, et al. Hepatitis E virus and the safety of plasma products: investigations into the reduction capacity of manufacturing processes. Transfusion 2016;56:383-91. [Crossref] [PubMed]
- Schlenke P. Pathogen inactivation technologies for cellular blood components: an update. Transfus Med Hemother 2014;41:309-25. [Crossref] [PubMed]
- Hauser L, Roque-Afonso AM, Beyloune A, et al. Hepatitis E transmission by transfusion of Intercept blood system-treated plasma. Blood 2014;123:796-7. [Crossref] [PubMed]
- Ruane PH, Edrich R, Gampp D, et al. Photochemical inactivation of selected viruses and bacteria in platelet concentrates using riboflavin and light. Transfusion 2004;44:877-85. [Crossref] [PubMed]
- Mohr H, Steil L, Gravemann U, et al. A novel approach to pathogen reduction in platelet concentrates using short-wave ultraviolet light. Transfusion 2009;49:2612-24. [Crossref] [PubMed]
- Berger K, Klein HG, Seitz R, et al. The Wildbad Kreuth initiative: European current practices and recommendations for optimal use of blood components. Biologicals 2011;39:189-93. [Crossref] [PubMed]
- Viral Safety Evaluation of Biotechnology Products derived from Cell Lines of Human or Animal Origin Q5A(R1). 1999. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q5A_R1/Step4/Q5A_R1__Guideline.pdf
- What is Necessary to Realize the Promise of Advanced Therapy Medicinal Products. 2016. Available online https://www.lifescienceleader.com/docpreview/what-are-the-key-trends-in-global-biopharmaceutical-manufacturing-for-0001/22d40354-3424-4802-8dc6-340834d3a7a5
- Santayana G. The Life of Reason. Amherst: Prometheus Books, 1998:504.
- Ma H, Galvin TA, Glasner DR, et al. Identification of a novel rhabdovirus in Spodoptera frugiperda cell lines. J Virol 2014;88:6576-85. [Crossref] [PubMed]
- Zhang W, Li L, Deng X, et al. Viral nucleic acids in human plasma pools. Transfusion 2016;56:2248-55. [Crossref] [PubMed]
Cite this article as: Kreil TR. Building blocks of the viral safety margins of industrial plasma products. Ann Blood 2018;3:14.


