Diagnosis and management of von Willebrand disease in Slovakia
Introduction
Von Willebrand disease (VWD) is usually reported to be “the most common human inherited bleeding disorder”, actually found in approximately 1% of the general population (1-3). The disorder was first described in 1926 by Erik von Willebrand, who recognized that it differed from hemophilia and named it “hereditary pseudohemophilia” (4). The factor in plasma that corrects the disease was not identified until many years later and was called von Willebrand factor (VWF). VWF is a complex plasma protein with multiple functions, which overall contribute to the formation of a platelet thrombus at sites of injury to help prevent blood loss. VWF accomplishes this major haemostasis function by anchoring platelets to sites of vascular injury as well binding to factor VIII (FVIII), thus protecting FVIII from degradation and delivering it to sites of vascular injury (5,6). The disease is characterized mainly by mucosa-associated bleeding and bleeding after surgery and trauma. The diagnosis is based on a personal or family history of bleeding and laboratory evidence of abnormalities in VWF, factor VIII, or both. Affected patients essentially have reduced levels of functional VWF, and various types of VWD can be distinguished on the basis of phenotypic characteristics. Deficiency of VWF results in a bleeding disorder that varies in severity according to the degree of deficiency and the specific characteristics of the molecule (7,8). Clinical expression of VWD is usually mild in type 1, with increasing severity in type 2 and type 3. In general, the severity of bleeding correlates with the degree of the reduction of VWF and FVIII. Mucocutaneous bleeding (epistaxis especially during childhood, easy bruising) is a typical, prominent manifestation of the disease and may affect the quality of life. However, the rate of spontaneous bleeding may be low even in patients with severe VWF deficiency (9). This review summarizes our management of patients with VWD in Slovakia based on the literature and our experience.
Methods
This study design is retrospective and observational. The population of VWD patients were recruited from a regional registry at the Martin University Hospital and National Registry of Congenital Bleeding Disorder at Bratislava University Hospital, which contain comprehensive demographic and clinical data. However, for scientific purposes data was then de-identified by the authors and handled in this manner. The regional registry is a database that accumulates clinical data about patients with coagulation disorders from all across Slovakia both for clinical and scientific purposes. As soon as a patient is diagnosed with bleeding disorder, he/she is being included in the registry.
Results
Organization of patients with VWD in Slovakia
The National Centre of Haemostasis and Thrombosis in Martin is one of the national haemophilia treatment centres in Slovakia. Together with the National Haemophilia Centre in Bratislava, our centre provides comprehensive care to patients with bleeding disorders. However, VWF multimer analysis and genetic testing is performed only in Martin. These two Slovakian centres provide services to more than 2,100 patients, both paediatric and adult, from all over Slovakia who have been diagnosed with a bleeding disorder. They offer state-of-the-art comprehensive clinical care, education to patients and their families, and accessibility to clinical research projects that are oriented to improving the lives of people with bleeding disorders. Additionally, 62 local haemophilia centres help to improve care of these patients at a regional level.
Project description: “Genetic Background and Hemostatic Changes in the Patients with von Willebrand Disease”
In order to better explore the diagnosis of VWD in Slovakia, a study “Genetic Background and Haemostatic Changes in the Patients with VWD” has been initiated. The prominent aim of the proposed project is the correlation of laboratory results and genetic analysis with the clinical course of the disease, and their use for the indication, monitoring and subsequent individual management of substitution treatment of individuals with VWD.
Epidemiology
Referral-based prevalence is the number of patients seen at specialized centres divided by the total population served by those centres. Figure 1 (per 100,000 inhabitants) shows prevalence ranging from 1.0 in Ukraine and Russia to 16.3 in the UK. The true prevalence of VWD is probably higher than suggested by available estimates, due to misdiagnosis or misrecognition of VWD. The referral-based prevalence per 100,000 inhabitants is 11.2 in Slovakia, a figure that is relatively high according to published referral-based prevalence around the world (10). The total Slovak population is 5,443,583 and the total number of patients with VWD in 2017 is 610 according to the National Registry of Congenital Bleeding Disorders in Slovakia, which is run by the National Haemophilia Centre, University Hospital and Medical School of Comenius University in Bratislava. Type 1 patients represented 65% (n=397), type 2A 20.4% (n=124), type 2B 3.0% (n=18), type 2M 1.3% (n=8), type 2N 2.0% (n=12) and type 3 8.4% (n=51), see Table 1.
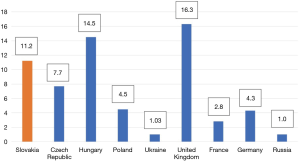
Table 1
| VWD type | Type 1 | Type 2A | Type 2B | Type 2M | Type 2N | Type 3 |
|---|---|---|---|---|---|---|
| Number of patients (%) | 397 [65] | 124 (20.4) | 18 (3.0) | 8 (1.3) | 12 (2.0) | 51 (8.4) |
VWD, von Willebrand disease.
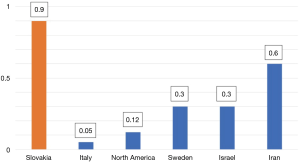
The world-wide prevalence figures for type 3 VWD vary substantially as shown in Figure 2 (10). It is surprising that Slovakia has one of the highest prevalence of VWD type 3 in the world.
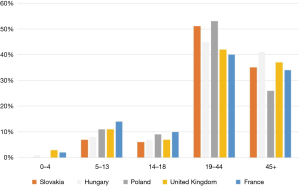
The age distribution of patients with VWD is shown in Figure 3 (10). Age distribution is very similar around the world. Most patients are of working age.
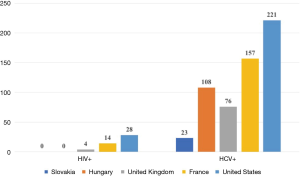
The number of patients with HIV and hepatitis C is shown in Figure 4 (10). It is pleasing to state that Slovakia has no HIV affected VWD patient. Hepatitis C was diagnosed in 23 VWD patients.
Diagnosis of VWD in Slovakia
Diagnosis in general follows guidelines outlined by the European Group on VWD, British Committee for Standards in Haematology and Slovak Society on Haemostasis and Thrombosis (11,12). Diagnosis of VWD is primarily based on VWF antigen (VWF:Ag) and on the VWF ristocetin cofactor (VWF:RCo) activity assay. The laboratory at the National Centre of Haemostasis and Thrombosis in Martin provides factor VIII activity (FVIII:C), VWF:Ag, VWF:RCo, VWF collagen binding (VWF:CB) and VWF multimers together as a “VWD profile”. Blood type is also obtained, as VWF levels vary by blood type. Our centre also performs low-dose ristocetin-induced platelet aggregation in case of suspicion of type 2 VWD. As mentioned above, the National Centre of Haemostasis and Thrombosis in Martin has this year begun sequencing of the whole VWF gene in all our patients.
Most patients have had preliminary screening tests performed prior to arrival in our department, including CBC (complete blood count), PT (prothrombin time), and APTT (activated partial thromboplastin time). If these have not been performed, we include them with our workup, along with a thrombin time (TT) and fibrinogen level. Patients are diagnosed with type 3 VWD if there is undetectable VWF:Ag and VWF:RCo. The VWF:Ag and VWF:RCo are rechecked to confirm low levels before a definitive diagnosis is made. Type 1 VWD is diagnosed if the VWF:Ag and VWF:RCo are <30 IU/dL, with relative concordance (i.e., RCo/Ag >0.6, while those with VWF:Ag and VWF:RCo between 30 and 50 IU/dL with concordance are labelled as “deficiency of VWF”.
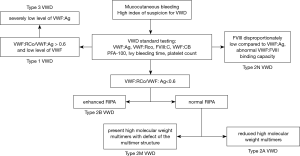
Additional tests, needed to distinguish and classify type 2 VWD (RCo/Ag <0.6), include VWF multimer analysis, VWF:CB, VWF-FVIII binding and ristocetin induced platelet aggregation (RIPA). The use of VWF:RCo and VWF:CB together improve the ability to detect type 2 variants. The VWF:CB is more accurate than the VWF:RCo in differentiating mild type 1 VWD from normal and in accurately assessing the level in severe types 1 or 3 disease. Type 2A VWD is identified by loss of high-molecular-weight multimers (HMW) VWF on multimer analysis. The laboratory finding of type 2B is the heightened RIPA with mild to moderate thrombocytopenia and the absence of HMW in plasma. Platelet type of VWD is often mistakenly diagnosed as type 2B VWD for the similarities between these two conditions. We hope that genetic testing in all our VWD patients will prevent such errors. In type 2M VWD the VWF multimer distribution is normal, but decreased interaction of VWF with GP1bα; therefore, the VWF:RCo assay is reduced, leading to a low VWF:RCo/VWF:Ag ratio (<0.6). Type 2N is characterized with mildly reduced or normal levels of VWF:Ag and VWF:RCo and a normal multimers, but low plasma levels of FVIII (typically 5–40 U/dL), which result from the decreased plasma half-life of FVIII, which cannot bind to VWF. The definitive diagnosis of VWD type 2N is made by measuring the VWF:FVIII-binding capacity. Our VWD testing algorithm is shown in Figure 5.
Treatment of VWD
Treatment also follows guidelines outlined by the European Group on VWD, British Committee for Standards in Haematology and Slovak Society on Haemostasis and Thrombosis (11,12). There are two treatments of choice in VWD, non-concentrate therapies (desmopressin, tranexamic acid) and transfusion therapy with plasma-derived concentrates FVIII/VWF or concentrates containing either high purity VWF alone.
Most patients with type 1 VWD receive desmopressin as first line therapy. This may be administered intravenously or via intranasal spray. The exception is those patients with clearance defects (type 1C VWD) who may experience an initial rise in VWF:Ag and VWF:RCo in response to desmopressin but which then is rapidly cleared. In these patients, VWF-containing concentrates are chosen to achieve sustained therapeutic VWF levels. Some type 2 patients may also receive desmopressin for minor bleeds such as epistaxis, in particular type 2A patients.
Antifibrinolytic drugs are used for mucosal bleeding, particularly following tonsillectomy or tooth extraction or for women with menorrhagia. Oral administration is used for menorrhagia and post-tonsillectomy surgical prophylaxis, while topical administration is used for prevention or treatment of bleeding following tooth extraction. For minor dental procedures, treatment is given for 5–7 days and up to 14 days for multiple molar extractions; for tonsillectomy, antifibrinolytic therapy is generally continued for up to 14 days or until the eschar is shed.
VWF-containing concentrates (plasma-derived concentrates containing FVIII/VWF or high-purity VWF concentrate) are the primary therapy for patients with type 2 and type 3 VWD, as well as for the more severely affected type 1 patients. At this time, plasma derived concentrates are used exclusively. Haemate-P®, Willate® and Willfact® are licensed plasma-derived concentrates for treatment of VWD in the Slovakia.
Our local practice with replacement therapy during surgery, dental extraction and delivery or puerperium is identified in Table 2.
Table 2
| Parameter | Major surgery | Minor surgery | Spontaneous bleeding episodes | Dental extractions | Delivery and puerperium |
|---|---|---|---|---|---|
| Doses VWF/FVIII concentrate | loading dose 50–60 U/kg and maintenance dose 25 U/kg every to 24 hours | 30–60 U/kg daily or every other day | single or daily dose 20–60 U/kg | single dose 30 U/kg | 50 U/kg daily or every other day |
| Therapeutic goal | through VWF:RCo and FVIII:C >50 IU/dL for 7–14 days | FVIII:C >30 IU/dL until healing is complete (2–4 days) | FVIII:C >30 IU/dL until bleeding stops (2–4 days) | FVIII:C >50 IU/dL for 12 hours | FVIII:C >50 IU/dL for 3–4 days |
| Safety parameter | do not exceed VWF:RCo 200 IU/dL or FVIII 250–300 IU/dL | – | – | – | – |
VWD, von Willebrand disease; VWF, von Willebrand factor.
Conclusions
The prevalence of VWD in the Slovak Republic is approximately 11.2 cases per 100,000 people, but this is probably still underestimated due to the fact that many patients, particularly those with mild disease, continue to obtain care through primary care physician. VWD diagnosis and management is best performed through hematology specialists familiar with the complexities of this condition, such as those in the hemophilia treatment centres. Improving the diagnosis of VWD is one of the goals of the study “Genetic Background and Hemostatic Changes in the Patients with VWD”, but much work remains to be done regarding treatment and quality of life for affected patients.
Acknowledgments
Funding: The study was supported by grants VEGA 1/0187/17.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Emmanuel J. Favaloro) for the series “Diagnosis and Management of von Willebrand Disease: Diverse Approaches to a Global and Common Bleeding Disorder” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2018.01.03). The series “Diagnosis and Management of von Willebrand Disease: Diverse Approaches to a Global and Common Bleeding Disorder” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and individual informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rodeghiero F, Castaman G, Dini E. Epidemiological investigation of the prevalence of von Willebrand's disease. Blood 1987;69:454-9. [PubMed]
- Leebeek FW, Eikenboom JC. Von Willebrand's disease. N Engl J Med 2016;375:2067-80. [Crossref] [PubMed]
- Echahdi H, El Hasbaoui B, El Khorassani M, et al. Von Willebrand's disease: case report and review of literature. Pan Afr Med J 2017;27:147. [Crossref] [PubMed]
- Von Willebrand EA. Hereditar pseudohemofili. Fin Lakaresallsk Handl 1926;68:87-112.
- Yee A, Kretz CA. Von Willebrand factor: form for function. Semin Thromb Hemost 2014;40:17-27. [PubMed]
- Springer TA. Von Willebrand factor, Jedi knight of the bloodstream. Blood 2014;124:1412-25. [Crossref] [PubMed]
- Mannucci PM. Treatment of von Willebrand's disease. N Engl J Med 2004;351:683-94. [Crossref] [PubMed]
- Castaman G, Tosetto A, Federici AB, et al. Bleeding tendency and efficacy of anti-haemorrhagic treatments in patients with type 1 von Willebrand disease and increased von Willebrand factor clearance. Thromb Haemost 2011;105:647-54. [Crossref] [PubMed]
- Zimmerman TS, Ratnoff OD, Powell AE. Immunologic differentiation of classic hemophilia (factor 8 deficiency) and von Willebrand’s disease, with observations on combined deficiencies of antihemophilic factor and proaccelerin (factor V) and on an acquired circulating anticoagulant against antihemophilic factor. J Clin Invest 1971;50:244-54. [Crossref] [PubMed]
- Report on the WFH Annual Global Survey 2015 [online]. [cit. 2017-30-09]. Available online: http://www1.wfh.org/publication/files/pdf-1669.pdf
- Castaman G, Goodeve A, Eikenboom J. Principles of care for the diagnosis and treatment of von Willebrand disease. Haematologica 2013;98:667-74. [Crossref] [PubMed]
- Laffan MA, Lester W, O'Donnell JS, et al. The diagnosis and management of von Willebrand disease: a United Kingdom Haemophilia Centre Doctors Organization guideline approved by the British Committee for Standards in Haematology. Br J Haematol 2014;167:453-65. [Crossref] [PubMed]
Cite this article as: Kubisz P, Sokol J, Simurda T, Plamenova I, Dobrotova M, Holly P, Skornova I, Stasko J. Diagnosis and management of von Willebrand disease in Slovakia. Ann Blood 2018;3:9.