
Von Willebrand disease in Iran: diagnosis and management
Introduction
von Willebrand disease (VWD) is the most common inherited bleeding disorder with an estimated incidence of ~1% in the general population and a symptomatic prevalence of ~1 per 1,000 individuals (1,2). The disorder arises from defects in the structure or function of the plasma protein called von Willebrand factor (VWF), or from deficiencies in levels of plasma VWF. The disorder was first described in 1926 by Finnish physician Erik von Willebrand in several families with severe mucocutaneous bleeding (3,4). VWD is categorized into quantitative or qualitative defects. Types 1 and 3 represent partial quantitative deficiency and virtually complete deficiency of protein, respectively, while type 2 comprises a qualitative defect classified into four separate subtypes (2A, 2B, 2M, and 2N). Type 2A VWD is associated with selective deficiency of high molecular weight (HMW) VWF multimers, leading to defective VWF-dependent platelet adhesion. Type 2B VWD is also associated with decreased HMW multimers, but represents a gain of function increase in VWF-platelet interactions due to hyper-adhesive VWF. Type 2M VWD represents a loss-of-function VWF accompanied with normal VWF multimers and, and type 2N VWD involves abnormal binding of VWF to FVIII as also associated with a normal multimer pattern (5).
Patients with VWD present a highly variable clinical phenotype, ranging from an asymptomatic condition (mostly in type 1 VWD) to a severe life-threatening bleeding diathesis (mostly in type 3 VWD) (5). In Western countries, type 1 VWD is most frequent, whereas type 3 VWD is rare. However, due to the high rate of consanguinity, type 3 VWD is relatively more frequently seen in developing countries. Iran as a Middle Eastern country with a high rate of consanguineous marriages, therefore, has a relatively high incidence of type 3 VWD. In the current report, we review the prevalence, clinical presentations, diagnosis and management of VWD in Iran.
VWD in Iran
Iran as a Middle Eastern country with a population of about ~82 million is the 18th largest population in the world (6). Twenty-nine hemophilia treatment centers and 47 medical universities (according to data from the Iranian ministry of health) are responsible for education, management, and treatment of patients with VWD in Iran. Soon after the first diagnostic and treatment center for inherited bleeding disorders was established at the new-found Tehran University Clinical Haematology Department, Pahlavi (now Emam Khomeini) Hospital in 1965, the Iranian Hemophilia Society (IHS; a non-profit, non-governmental organization) was created in 1967 by families suffering from congenital hemorrhagic disorders, such as hemophilia. In addition to running other satellite treatment centers throughout the country, the IHS contributes to direct (face-to-face) and indirect education of patients with these bleeding disorders such as VWD, by publishing brochures, holding workshops, and lectures, etc. (7,8). Furthermore, these centers are responsible for improving patients’ access to treatment facilities (8). Currently, two hemophilia centers have well-equipped hemostasis laboratories, the Iranian Comprehensive Hemophilia Care Center (ICHCC) managed by the IHS in Tehran, and the Shiraz University Medical School Center at the Nemazee Hospital. The Iranian Blood Transfusion Organization (IBTO) also has well-equipped diagnostic laboratories for hemostatic disorders, both in Tehran and in its Provincial Transfusion Centers throughout Iran. Generally, the medical universities represent the main centers for the treatment of VWD (and other bleeding disorders), as well as implementing plans for prophylactic treatment (8). Some of these have been equipped with specialized laboratories for the diagnosis of hemostatic disorders. Two Comprehensive Care Centers affiliated to the Iranian hemophilia society are also available for treatment of coagulation disorders (7).
In spite of impressive developments in the diagnosis of VWD in Iran, there is unfortunately no exact information about the true number of patients with VWD. Recently, the Iranian Ministry of Health has established a database to record patients who receive treatment for bleeding disorders. The IHS has another longer-standing database which includes many additional patients. This discrepancy is due to the presence of patients with a mild clinical phenotype registered on the IHS database that do not normally require treatment and therefore are not included in Ministry of Health database. Based on the IHS database, 10,944 patients have been registered with hemostatic disorders, while only 9,500 patients are registered on the Iranian Ministry of Health database. A total of 1,617 patients in the IHS database are affected by VWD. The national registry for hemostatic disorders in the IHS provides reliable information, especially regarding the number of patients with congenital bleeding disorders such as VWD. However, the lack of well-equipped laboratories in some parts of the country may cause imprecision in the number of well characterized patients, especially for those where relatively sophisticated laboratory diagnosis approaches are required, such as VWD and congenital fibrinogen disorders. To resolve this issue, the Iranian Ministry of Health aims to develop 10 major well-equipped hemophilia treatment centers, mostly to be located in general hospitals for the diagnosis and comprehensive management of hemostatic disorders. It is to be hoped that, once it is implemented, this initiative will result in even better support for hemophilia societies and their patients in the Provinces outside the capital.
Prevalence of VWD in Iran
Based on the IHS report, the total number of patients with hemostatic disorders in Iran is 10,944 (a prevalence of 131 per million), while the number of registered patients with VWD is 1,617 and 1,516 according to the IHS and annual global survey report of World Federation of Hemophilia (WFH), respectively (a prevalence of ~19 per million) (9). Of the 1,617 patients with VWD, 12 and 1 are from Afghanistan and Iraq, respectively and the rest of them are from Iran. However, it seems that these data may not present the exact number of the patients with VWD in Iran and some patients may be misdiagnosed or remain undiagnosed due to the mild bleeding phenotypes and absence of well-equipped hemostasis laboratories around the country. The diagnosis of VWD, especially type 1 and type 2, is difficult, requiring well-equipped laboratories and expert technical staff. Thus, these VWD types are expected to be underestimated. However, currently, some additional efforts have been made to improve this situation at the ICHCC, especially for those with type 2 VWD. Nevertheless, more than half of the registered patients with VWD are still not classified and therefore the exact prevalence of different types of VWD in Iran is unclear. In addition, insufficient public knowledge about abnormal bleeding and infrastructural health problems leads to under-diagnosis of VWD in Iran (especially type 1). In general, out of the 1,617 registered patients, 206 (12.7%) are listed as type 1, 199 (12.3%) as type 2, 304 (18.8%) as type 3, and 908 patients (56.1%) as unclassified. All 199 patients registered with type 2 VWD have been sub-characterized as type 2A VWD [55 (27.6%) patients], type 2B VWD [33 (16.5%) patients], type 2M VWD [40 (20.1%) patients], type 2N VWD [38 (19%) patients], type 2C VWF [4 (2%)], type 2D [2 (1%)], type Vicenza VWD [25 (12.5%)], and platelet-type VWD [2 (1%)]. The greatest number of patients with VWD has been identified in Tehran (the capital of Iran), Khorasan Razavi and Khuzestan, respectively (Figure 1).
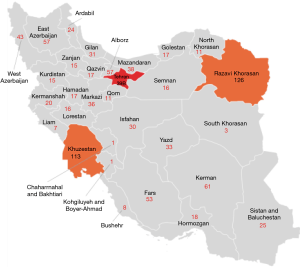
Overall, due to high rate of consanguineous marriage and autosomal recessive inheritance pattern of type 3, the prevalence of type 3 is around 4 per million in Iran, which overshadows that of Western European countries with a reported prevalence of ~0.45 per million (10).
Molecular basis of VWD in Iran
According to the International Society on Thrombosis and Hemostasis (ISTH)-Scientific and Standardization Committee (SSC) of VWF online database (http://ragtimedesign.com/vwf/mutation/), about 120 different gene defects have been reported in patients with type 3 VWD. These gene defects consist of missense, deletions, small insertions, nonsense and splice site mutations and most of them result in null alleles (11). Several molecular studies have been performed on Iranian patients with VWD. Most of these studies have focused on type 3 VWD (12). In one genetic study of type 2 VWD, a homozygous Cys410Ser mutation on D2 domain of VWF was identified (13). This mutation results in type 2A (IIC) VWD. In a study of 40 type 3 VWD patients with three different nationalities, consisting of 14 Iranians, 12 Italians, and 14 Indians patients, molecular characteristics of Iranian patients revealed, eight nonsense mutations (Q218X, R365X, E644X, Q706X, Q1311X, Q1346X, R1659X, E1981X), two missense mutations (D47H and C2174G), three frame shift mutations (1110-1G→A, 7674insC, 7683delT) and one deletion (788del24) (14), as summarized in Table 1.
Table 1
| Patients | Sex | Age (year) | VWF: Ag | Anti VWF anti-body | Consanguinity | Bleeding tendency | Nucleotide acid substitution | Amino acid substitution | Exon | Genotype |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 8 | <1 | – | Yes | Mild | 139G→C | D47H | 3 | Homozygous |
| 2 | F | 26 | 2 | – | Yes | Severe | 652C→T | Q218X | 6 | Homozygous |
| 3 | F | 18 | <1 | – | Yes | Mild | 788del24 | 263del8 | 7 | Homozygous |
| 4 | F | 16 | 1 | – | Yes | Severe | 1093C→T | R365X | 9 | Homozygous |
| 5 | M | 19 | 2 | – | Yes | Severe | 1110-1G→A | FS | 10 | Homozygous |
| 6 | M | 21 | 2 | – | Yes | Severe | 1930G→T | E644X | 15 | Homozygous |
| 7 | F | 11 | 2 | – | Yes | Severe | 2116C→T | Q706X | 16 | Homozygous |
| 8 | F | 20 | <1 | – | Yes | Severe | 3931C→T | Q1311X | 28 | Homozygous |
| 9 | F | 52 | <1 | 10 | Yes | Severe | 4036C→T | Q1346X | 28 | Homozygous |
| 10 | F | 36 | 1 | – | Yes | Severe | 4975C→T | R1659X | 28 | Homozygous |
| 11 | M | 16 | <1 | – | Yes | Severe | 5941G→T | E1981X | 35 | Homozygous |
| 12 | F | 17 | 1 | – | Yes | Severe | 6520T→G | C2174G | 37 | Homozygous |
| 13 | M | 20 | 1 | – | Yes | Mild | 7674insC | FS | 45 | Homozygous |
| 14 | M | 24 | <1 | – | Yes | Severe | 7683delT | FS | 45 | Homozygous |
VWD, von Willebrand disease; FS, Frame shift; Del, Deletion; Ins, insertion.
Moreover, in the report of Cohan et al., some other mutations in Iranian patients have also been reported, including, a single 2365A>G mutation on exon 18 in Iranian patients with type 2N VWD. This mutation results in T789P amino acid substitution. A small deletion (3237delA at exon 25) was also reported in type 3 VWD (Table 2) (12).
Table 2
| Number | VWD type | Nucleotide acid substitution | Amino acid substitution | Exon |
|---|---|---|---|---|
| 1 | 3 | 191delG | G64AfsX19 | 3 |
| 2 | 3 | 788-811del | C263-E270del | 7 |
| 3 | 3 | 5941G>T | E1681X | 35 |
VWD, von Willebrand disease; FS, frame shift; Del, deletion.
In another study on 10 patients with type 3 VWD, several other VWF mutations were identified (Table 3) (11).
Table 3
| Patients | Sex | Age (year) | VWF: Ag | Consanguinity | Nucleotide acid substitution | Amino acid substitution | Exon | Genotype |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 24 | <1 | Yes | 5941G>T | E1981X | 35 | Homozygous |
| 2 | F | 25 | <1 | No | – | – | – | – |
| 3 | M | 35 | <1 | Yes | 2443G>C | Splice site | 19 | Homozygous |
| 4 | F | 21 | <1 | Yes | 310C>T | Q104X | 4 | Homozygous |
| 5 | M | 48 | <1 | Yes | 2377C>T | Q793X | 18 | Homozygous |
| 6 | F | 29 | <1 | Yes | 3237delA | P1079fsX39 | 25 | Homozygous |
| 7 | F | 55 | <1 | No | 2377C>T | Q793X | 18 | Homozygous |
| 2611G>C | G871R | 20 | Heterozygous | |||||
| 8 | F | 35 | <1 | Yes | 2377C>T | Q793X | 18 | Homozygous |
| 9 | M | 28 | <1 | Yes | Homozygous | |||
| 10 | M | 23 | <1 | Yes | 1110-1G>A | Splice site | 10 | Homozygous |
VWD, von Willebrand disease; FS, frame shift; Del, deletion.
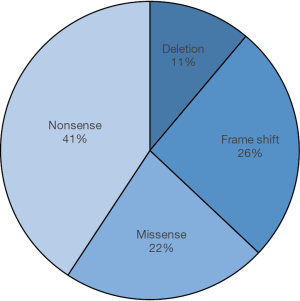
Additionally, a distinct mutation (Asp235Tyr) in GP1BA leading to platelet-type VWD (PT-VWD), with similar clinical and laboratory features to type 2B VWD, has also been reported (15) in two Iranian patients. This mutation generates hydrophobic tip to the extended B-switch loop of GPIba (15).Taken together, of 27 reported mutations in types 2 and 3 VWD and also in PT-VWD in Iran, scientists have identified 41% nonsense mutations, 22% missense mutations, 26% frame shifts and 11% deletions (Figure 2).
Clinical manifestations of VWD in Iran
The main clinical manifestation of VWD is mucocutaneous bleeding (16). As anticipated, deficiency of VWF disrupts primary hemostasis, typically leading to mucosal bleeding. However, in keeping with the biological heterogeneity of VWD, the spectrum of bleeding symptoms is variable in these patients, ranging from an asymptomatic condition (usually in type 1) to a severe clinical diathesis (mostly in type 3) (17). In a clinical and laboratory survey on 385 Iranian patients with type 3 VWD, epistaxis (77%) and menorrhagia (69%), both representing mucosal hemorrhages, were the most frequent clinical manifestations, and central nervous system bleeding (2%) and hematuria (1%) were rare symptoms (18). The results of another study demonstrated that about 70% of patients with type 3 VWD from different parts of Iran have experienced epistaxis, which in some cases was serious, and required replacement therapy (11). In women with bleeding disorders, menorrhagia is the most common clinical symptom (19). The results of a study on 56 women with hereditary bleeding disorders that experienced menorrhagia showed that 55.3% of them had VWD, with type 3VWD being the predominant type (20). The high prevalence of menorrhagia in VWF deficiency was also demonstrated in a study on women with menorrhagia in the north of Iran (21). The high prevalence of menorrhagia in patients with VWD emphasizes the necessity for increasing awareness of this disease, both in the public domain, but also among general practitioners and gynecology specialists, in order to achieve better management and more effective treatment of affected patients. Other common bleeding symptom in Iranian patients with type 3 VWD is oral cavity bleeding, which may results from teeth extraction or traumatic lesion on the tongue or lips (18). In a study of 50 patients with VWD in north-eastern Iran where subtypes were not determined, vast ecchymosis (76%) was noted as the most common symptom. In addition to this, menorrhagia, epistaxis, and post-dental extraction bleeding were reported as other common presentations (22). Muscle hematoma (52%) and spontaneous hemarthrosis (37%) are also relatively common in type 3 VWD, just as in patients with severe hemophilia A. Iliopsoas hemorrhage is known as a serious complication of bleeding disorders that may results in muscle dysfunction and femoral nerve paralysis. This complication was reported in a 20-year-old Iranian patient with type 3 VWD, who was well managed by Humate-P (CSL Behring, Marburg, Germany) (18,23).
VWF deficiency does not affected fertility and pregnancy in Iranian VWD women, as compared to normal women. Because of the previously noted inaccessibility of standard diagnostic tools in Iranian medical diagnostic laboratories and due to the inheritance pattern of type 3 VWD, most registered symptomatic patients with VWD are type 3, mainly due to the severity of the clinical phenotype (18). Available reports for types 1 and 2 VWD in Iranian patients are fewer, due to lower numbers of identified patients and the more sophisticated diagnostic investigation required for these VWD types. One 8-year-old patient with type 2A VWD had recurrent, heavy epistaxis and was confirmed to have a homozygous Cys410Ser mutation (13). It can be concluded that the most common clinical symptom in Iranian patients with VWD is epistaxis (Table 4).
Table 4
| Authors (reference) | Year of publication | Origin of study | No. of patients | Bleeding symptom | Prevalence [%] |
|---|---|---|---|---|---|
| Lak |
2000 | Not mentioned | 385 | Epistaxis | 269 [70] |
| Oral cavity bleeding | 296 [77] | ||||
| Hemarthrosis | 141 [37] | ||||
| Muscle hematoma | 200 [52] | ||||
| Gastrointestinal bleeding | 74 [19] | ||||
| 130 | Menorrhagia | 90 [69] | |||
| 203 | Post-operative bleeding | 83 [41] | |||
| 100 | Post-partum bleeding | 15 [15] | |||
| Mansouritorghabeh |
2013 | North-Eastern | 50 | Epistaxis | 43 [86] |
| Vast ecchymosis | 38 [76] | ||||
| Menorrhagia | 14 [63]* | ||||
| Post-dental extraction bleeding | 11 [85]* | ||||
| Gastrointestinal tract bleeding | 12 [24] | ||||
| Gum hemorrhage | 16 [32] | ||||
| Umbilical Cord bleeding | 2 [4] | ||||
| 17 | Post-operative bleeding | 11 [65] | |||
| Shahbazi |
2009 | Not mentioned | 10 | Epistaxis | 7 [70] |
| Oral cavity bleeding | 9 [90] | ||||
| Payandeh |
2013 | Kermanshah | 56 | Menorrhagia | 31 [55] |
*Among patients at risk.
Diagnosis of VWD in Iran
The diagnosis of VWD is based on clinical manifestations, family history, routine laboratory tests and specific assays. Laboratory practices which related to diagnosis and identification of VWD in Iran and its subtypes is somewhat different from standard recommended protocols. In Iranian laboratories, following evaluation of the patent’s past bleeding history and family history, the diagnosis of VWD is started via routine laboratory tests. Initially, a complete blood count (CBC), particularly platelet count, is evaluated. If the platelet count and morphology are normal, routine coagulation tests including prothrombin time (PT), activated partial thromboplastin time (aPTT) and also platelet function analysis (PFA) are recommended. The PT and aPTT are available in almost all government-funded and private centers. The majority of these centers are equipped with basic instrumentation and these two tests are usually performed by automated analyzers, while in less well-equipped centers, tests may still be based on manual methods. For specific assays, patients are referred to the centers that are specialized in the diagnosis of hemostatic and platelet disorders. The diagnostic panel for VWD available in Iran includes VWF antigen (VWF:Ag), factor (F) VIII coagulant activity (FVIII:C), VWF Ristocetin Cofactor (VWF:RCo), Ristocetin Induced Platelet Aggregation (RIPA), VWF: Collagen Binding (VWF:CB) assay and VWF FVIII binding assay (VWF:FVIIIB) (Table 5). Several governmental and private centers perform VWF: Ag and FVIII: C, while only 3 centers perform VWF:RCo, VWF:CB, RIPA and VWF:FVIIIB. These centers, which include the ICHCC of the IHS, the Shiraz University Hemophilia Centre (SHS) and the IBTO hemostasis laboratories are considered as referral centers. The only center to have ever performed VWF multimer analysis is the ICHCC. In addition, Tehran has better equipped laboratories, including 4 private laboratories that perform VWF:Ag and FVIII:C assays. In the ICHCC laboratory, VWF:Ag is measured based on latex method, whereas the VWF:CB and VWF:FVIIIB assays are estimated based on enzyme-linked immunosorbent assays (ELISA). In addition, this center conducts VWF:RCo testing using an aggregometer. In RIPA, the ristocetin response is assessed in the presence of a range of ristocetin concentrations.
Table 5
| Laboratory assay | Routine coagulation laboratory tests | Specific assays | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT | aPTT | Platelet count | BT | PFA-100 | FVIII: C | VWF: Ag | VWF: RCo | VWF: CB | VWF: RCo to VWF: Ag | RIPA | ||
| Type 1 | Normal | Normal to prolonged | Normal | Prolonged | Normal or prolonged | Normal or low | Low | Low | Low | >0.7 | Normal or reduced | |
| Type 2A | Normal | Normal to prolonged | Normal | Prolonged | Prolonged | Normal or low | Normal or low | Very low | Low | <0.7 | Normal or reduced | |
| Type 2B | Normal | Normal to prolonged | Normal to low | Prolonged | Prolonged | Normal or low | Normal or low | Low | Low | <0.7 | Response to low concentration of ristocetin | |
| Type 2M | Normal | Normal to prolonged | Normal | Prolonged | Prolonged | Normal or low | Normal or low | Low | Low or normal | Low (<0.7) or normal | Normal or reduced | |
| Type 2N | Normal | Prolonged | Normal | Normal | Normal | Low | Normal or low | Normal or low | Normal or low | >0.7 | Normal | |
| Type 3 | Normal | Prolonged | Normal | Prolonged | Prolonged | Low | Undetected |
Undetected |
Undetected |
Not calculated | Absent | |
PT, prothrombin time; BT, bleeding time; aPTT, activated partial thromboplastin time; PFA, platelet function analysis; VWF:Ag, von Willebrand factor: antigen; VWF:RCo, von Willebrand factor: ristocetin cofactor; VWF:CB, von Willebrand factor: collagen binding; RIPA, ristocetin-induced platelet aggregation.
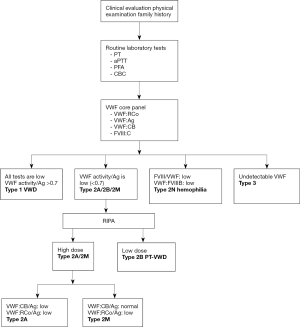
The approach for diagnosis of different types of VWD is as follow (Figure 3): type 1 VWD has a decrease in VWF measured by all VWF assays and the ratio of VWF activity assay/VWF antigen is expected to be >0.7. Type 3 VWD is determined by total loss of VWF (the results of all VWF test results are close to 0 U/dL). In VWF type 2 and its subtypes (except for type 2N), the VWF:RCo/VWF:Ag ratio <0.7 is highly suggestive. Type 2A VWD is determined by the relative reduction of all VWF activity assays (i.e., low VWF:RCo/Ag and low VWF:CB/Ag ratios). Type 2B VWD has a similar VWF activity test pattern to type 2A, but also required for its diagnosis is responsiveness to a low concentration of ristocetin (‘increased RIPA’). In Type 2M VWD the VWF:CB/Ag ratio may be normal. Type 2N VWD is identified by decreased FVIII/VWF and defined by decreased binding affinity of VWF for FVIII, which is determined by the VWF:FVIIIB assay.
Management of VWD in Iran
Treatment of VWD is dependent on the type and severity of the disorder. In general, on-demand therapy is the mainstay of treatment in VWD. Several therapeutic choices including desmopressin (1-deamino-8-D-arginine vasopressin) (DDAVP), adjuvant agents, cryoprecipitate, FFP, intermediate purity FVIII/VWF concentrate, high purity FVIII/VWF concentrate, recombinant VWF, and platelet concentrate are available for management of patients with VWD around the world (24,25), while in Iran desmopressin, FVIII/VWF concentrate (Humate-P and Wilate), cryoprecipitate and adjuvant agents (tranexamic acid) are typically available. Desmopressin is the main therapeutic choice for less severe types of VWD, especially type 1 and some patients with type 2 VWD, while replacement therapy is the more suitable choice in patients with severe forms of VWD, including type 3VWD and most patients with type 2 VWD, excepting type 2B cases. Long-term prophylaxis is available for severe form of VWD with life-threatening hemorrhages, particularly type 3 VWD (25-28).
Desmopressin
Desmopressin (DDAVP) is used as a therapeutic choice in most patients with type 1 VWD, possible type 1 VWD and some patients with type 2 VWD. There is a limitation in repeated administration of the drug because of depletion of FVIII and VWF storage. The drug can increase the FVIII and VWF levels 3 to 5 times after 30-minute treatment (25-28). DDAVP (Ferring GmbH, Kiel, Germany; Kedrion, Barga, Italy) can be administrated intravenously, intranasal or subcutaneously; however in Iranian patients, is most often given intravenously in a standard dose of 0.3 µg/kg (12). Based on severity of disease, drug injection may be repeated every 12 to 24 h. Desmopressin is usually avoided in cases of type 2B VWD, for fear of causing rapid clearance of platelets and thrombocytopenia.
Cryoprecipitate
Cryoprecipitate is used for many years as the main therapeutic choice for management of patients with VWD. This product contains VWF, FVIII, fibrinogen, FXIII, fibronectin and was administrated every 12–24 h to stop or control bleeding in patients with VWD. In general, large amount of cryoprecipitate (several bags of cryoprecipitate) are required for VWD management. Unfortunately, despite strict criteria for admission of donors and checking for infectious agents in donor blood samples before preparing cryoprecipitate in the Iranian blood transfusion centers, the risk of transmission of blood-borne diseases remain a residual problem for patients, because there is not protocol for virus-inactivation of cryoprecipitate in Iran. However, Thierry Burnouf, et al. have developed an ingenious protocol for Solvent-Detergent, virally inactivated pools of cryoprecipitate in a closed, multiple bag system for use in developing countries, which may turn out to be of interest in the future (12).
In a large study of 385 Iranian patients with type 3 VWD, the prevalence of HBs-Ag, Anti-HBs, anti-HCV, and anti-HIV was 2%, 50%, 55% and 1%, respectively (18). In fact, cryoprecipitate is primarily used in Iran as a rapid source of fibrinogen, to enhance levels to >1 g/L in dysfibrinogenemia and in acquired hypofibrinogenemia, which may develop in cases following massive transfusion and disseminated intravascular coagulation (DIC) (29,30). There are different recommendations for the use of cryoprecipitate in the treatment of inherited bleeding disorders such as VWD in different countries; for example, based on a circular with information regarding the indications for the use of human blood and blood components, published in the USA, whenever virus-inactivated factor concentrates are not available, cryoprecipitate is used as an alternative for the treatment of VWD (31). This product remains in use for the treatment of VWD in developing countries due to its broad availability and low cost.
Factor concentrates
Replacement therapy with human plasma products containing FVIII/VWF is the mainstay of management in patients unresponsive to desmopressin and is also indicated for long-term therapy. Several concentrates are available for treatment of VWD. These concentrates are given to patients with type 3 VWD, most patients with type 2 VWD, and a number of patients with type 1 VWD (32). Humate-P (CSL Behring, Marburg, Germany) and Wilate (Octapharma Pharmazeutika Produktionsges, Vienna, Austria) are used for treatment of VWD in Iranian patients. Humate-P was the first plasma-derived, intermediate-purity FVIII/VWF concentrates introduced for on-demand therapy and long-term prophylaxis in patients with VWD or hemophilia in Iran (32). This product is often used for Iranian patients unresponsive to desmopressin (32). The quantity and quality of the VWF, such as the composition of the HMW Multimers in this product is similar to normal plasma. No incident of blood-borne viral disease has hitherto been attributed to this product, and treatment-related adverse events are rare. Humate-P is administrated intravenously. The average ratio of VWF:RCo to FVIII:C in Humate-P is approximately 2.4:1 (32). Dose recommendation and duration of Humate-P therapy depends on the type and severity of disorder, the prophylaxis regimen, and the type of surgical intervention. However, the recommended dose in Iranian patients is 30–40 IU VWF:RCo per kg for minor surgery, delivery, and postpartum treatment, and 40–50 IU VWF:RCo per kg for major surgery and major bleeds (32). Wilate, a newly imported product in Iran, is a high-purity, double independent virus inactivated FVIII/VWF concentrate. The ratio of functional of VWF and FVIII in this concentrate is close to normal plasma. In addition, the VWF multimeric pattern of Wilate is relatively similar to normal plasma. However, the level of highest molecular weight multimers is relatively less than normal plasma (32). Unlike Humate-P, Wilate is not indicated for patients with hemophilia A due to the relatively lower amount of FVIII. The recommended dose of Wilate is 20–40 IU VWF:RCo per kg and 40–60 IU VWF:RCo per kg in minor hemorrhages and major hemorrhages, respectively. The therapeutic goal of these doses are >30% and >50% VWF:RCo and FVIII activity, respectively. In addition, the recommended dose of Wilate is 30–60 IU VWF:RCo per kg and 40–60 IU VWF:RCo per kg in minor and major surgeries, respectively, with therapeutic goal of VWF:RCo peak level of 50% and 100% after loading factor, respectively. It must be noted that the trough concentration is >30% and 50% during maintenance doses for major and minor surgery, respectively (32).
Adjuvant therapies
Anti-fibrinolytic therapy with tranexamic acid is also effective when it is used for the management of mucosal hemorrhagic symptoms in patients with VWD. It is believed that some common manifestations of VWD, such as epistaxis and menorrhagia, at least in part, can be due to the rich fibrinolytic activity of mucosal tracts. Tranexamic acid is a synthetic lysine analog that inhibits fibrinolysis by preventing the formation of plasmin and/or plasminogen (without changing the plasma level of VWF) (32). The recommended dose for patients with VWD is 25 mg/kg every 8 h. Tranexamic acid, as used in Iran, is domestically manufactured (32).
Persistent, severe menorrhagia in cases of VWD can also be moderated or even entirely resolved with the judicious use of oral contraceptive pills, which suppress pituitary gonadotrophin and prevent ovulation, or else, Progestins.
Prophylaxis
Although prophylaxis is not routinely used in patients with VWD, in patients with severe forms of VWD (usually type 3) and other VWD patients with recurrent bleeds, prophylaxis can be considered in order to prevent life-threatening hemorrhages and to control recurrent mucosal and joint bleeds. Short-term prophylaxis is also often used for surgery or invasive procedures to prevent excessive bleeding (32). The following patients may require secondary long-term prophylaxis (33,34):
- Patients with very low levels of VWF activity (<10 IU/dL) that present with recurrent gastro-intestinal (GI) bleeding.
- Women with repeated episodes of menorrhagia, unresponsive to desmopressin or tranexamic acid.
- Children with repeated epistaxis that leads to anemia.
- Patients with severe forms of VWD, accompanied with very low levels of FVIII (usually less than 5 IU/dL), that may have joint bleeding.
Because FVIII and VWF levels are corrected spontaneously during pregnancy in women with type 1 VWD, treatment or prophylaxis of these patients may not be necessary. There is not definitive schedule for support of Iranian patients by prophylaxis and, prophylaxis should only be used in select situations, such as type 3 VWD that present with ICH or sever GI bleeding, and in women with a history of severe bleeding during pregnancy. Prophylaxis can be used with 30 IU VWF:RCo per kg, 2 to 3 times a week (33,34).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Emmanuel J. Favaloro) for the series “Diagnosis and Management of von Willebrand Disease: Diverse Approaches to a Global and Common Bleeding Disorder” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2018.01.01). The series “Diagnosis and Management of von Willebrand Disease: Diverse Approaches to a Global and Common Bleeding Disorder” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- James PD, Lillicrap D, Mannucci PM. Alloantibodies in von Willebrand disease. Blood 2013;122:636-40. [Crossref] [PubMed]
- Bowman M, Hopman W, Rapson D, et al. The prevalence of symptomatic von Willebrand disease in primary care practice. J Thromb Haemost 2010;8:213-6. [Crossref] [PubMed]
- Lillicrap D. Translational medicine advances in von Willebrand disease. J Thromb Haemost 2013;11:75-83. [Crossref] [PubMed]
- Von Willebrand EA. Hereditar pseudohemofili. Finska Lakaresallskapets Handlingar 1926;LXVII:87-112.
- De Meyer SF, Deckmyn H, Vanhoorelbeke K. von Willebrand factor to the rescue. Blood 2009;113:5049-57. [Crossref] [PubMed]
- World factbook/Iran. Available online: https://www.cia.gov/library/publications/resources/the-world-factbook/geos/ir.html
- Available online: www.hemophilia.org.ir
- Eshghi P, Mahdavi-Mazdeh M, Karimi M, et al. Haemophilia in the developing countries: the Iranian experience. Arch Med Sci 2010;6:83. [Crossref] [PubMed]
- World Federation of Hemophilia—the report of Annual Global Survey of 2015. Available online: https://www1.wfh.org/publication/files/pdf-1669.pdf
- Mannucci P, Bloom A, Larriel M, et al. Atherosclerosis and von Willebrand factor. Br J Haematol 1984;57:163-9. [Crossref] [PubMed]
- Shahbazi S, Mahdian R, Ala F, et al. Molecular characterization of Iranian patients with type 3 von Willebrand disease. Haemophilia 2009;15:1058-64. [Crossref] [PubMed]
- Cohan N, Karimi M. Diagnosis and management of von Willebrand disease in Iran. Semin Thromb Hemost 2011;37:602-6. [Crossref] [PubMed]
- Enayat M, Ravanbod S, Rassoulzadegan M, et al. Identification of a homozygous Cys410Ser mutation in the von Willebrand factor D2 domain causing type 2A (IIC) von Willebrand disease phenotype in an Iranian patient. Haemophilia 2013;19:e261-4. [Crossref] [PubMed]
- Baronciani L, Cozzi G, Canciani MT, et al. Molecular defects in type 3 von Willebrand disease: updated results from 40 multiethnic patients. Blood Cells Mol Dis 2003;30:264-70. [Crossref] [PubMed]
- Enayat S, Ravanbod S, Rassoulzadegan M, et al. A novel D235Y mutation in the GP1BA gene enhances platelet interaction with von Willebrand factor in anIranian family with platelet-type von Willebrand disease. Thromb Haemost 2012;108:946-54. [Crossref] [PubMed]
- Boehlen F, Robert-Ebadi H, de Moerloose P. Von Willebrand disease: a common and unrecognized bleeding disorder. Rev Med Suisse 2007;3:346-50. [PubMed]
- Werner EJ, Broxson EH, Tucker EL, et al. Prevalence of von Willebrand disease in children: a multiethnic study. J Pediatr 1993;123:893-8. [Crossref] [PubMed]
- Lak M, Peyvandi F, Mannucci P. Clinical manifestations and complications of childbirth and replacement therapy in 385 Iranian patients with type 3 von Willebrand disease. Br J Haematol 2000;111:1236-9. [Crossref] [PubMed]
- Bevan JA, Maloney KW, Hillery CA, et al. Bleeding disorders: a common cause of menorrhagia in adolescents. J Pediatr 2001;138:856-61. [Crossref] [PubMed]
- Payandeh M, Rahimi Z, Kansestani AN, et al. Clinical features and types of Von Willebrand disease in women with menorrhagia referred to Hematology Clinic of Kermanshah. Int J Hematol Oncol Stem Cell Res 2013;7:1-5. [PubMed]
- Janbabaei G, Borhani S, Rashidi M, et al. Frequency of Bleeding Disorders in Women Presenting Menorrhagia in the North of Iran. Blood 2011;118:4660.
- Mansouritorghabeh H, Manavifar L, Banihashem A, et al. An investigation of the spectrum of common and rare inherited coagulation disorders in North-Eastern Iran. Blood Transfusion 2013;11:233. [PubMed]
- Keikhaei B, Shirazi AS. Spontaneous iliopsoas muscle hematoma in a patient with von Willebrand disease: a case report. J Med Case Rep 2011;5:274. [Crossref] [PubMed]
- Rodeghiero F, Castaman G, Tosetto A. How I treat von Willebrand disease. Blood 2009;114:1158-65. [Crossref] [PubMed]
- Sadler J, Mannucci P, Berntorp E, et al. Impact, diagnosis and treatment of von Willebrand disease. Thromb Haemost 2000;84:160-74. [PubMed]
- Federici AB, Mazurier C, Berntorp E, et al. Biologic response to desmopressin in patients with severe type 1 and type 2 von Willebrand disease: results of a multicenter European study. Blood 2004;103:2032-8. [Crossref] [PubMed]
- Mannucci PM. How I treat patients with von Willebrand disease. Blood 2001;97:1915-9. [Crossref] [PubMed]
- Mannucci P, Canciani M, Rota L, et al. Response of factor VIII/von Willebrand factor to DDAVP in healthy subjects and patients with haemophilia A and von Willebrand's disease. Br J Haematol 1981;47:283-93. [Crossref] [PubMed]
- Erber WN, Perry DJ. Plasma and plasma products in the treatment of massive haemorrhage. Best Pract Res Clin Haematol 2006;19:97-112. [Crossref] [PubMed]
- O'shaughnessy D, Atterbury C, Bolton Maggs P, et al. Guidelines for the use of fresh‐frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol 2004;126:11-28. [Crossref] [PubMed]
- Nascimento B, Goodnough L, Levy J. Cryoprecipitate therapy. Br J Anaesth 2014;113:922-34. [Crossref] [PubMed]
- Mannucci PM, Chediak J, Hanna W, et al. Treatment of von Willebrand disease with a high-purity factor VIII/von Willebrand factor concentrate: a prospective, multicenter study. Blood 2002;99:450-6. [Crossref] [PubMed]
- Federici A. Prophylaxis in patients with von Willebrand disease: who, when, how? J Thromb Haemost 2015;13:1581-4. [Crossref] [PubMed]
- Federici AB. Prophylaxis of bleeding episodes in patients with von Willebrand’s disease. Blood Transfusion 2008;6:s26. [PubMed]
Cite this article as: Dorgalaleh A, Tabibian S, Shams M, Ala F, Bahoush G, Jazebi M, Manafi R, Tavasoli B, Baghaipour MR. Von Willebrand disease in Iran: diagnosis and management. Ann Blood 2018;3:4.


