
Transfusion transmission of hepatitis E virus: an emerging issue
Hepatitis E virus (HEV) is the most recently identified human hepatitis virus. The virus was identified in 1983 using similar methods that had been used to successfully identify hepatitis A virus (HAV) about a decade earlier by Feinstone et al. at the National Institutes of Health (1). Balayan collected fecal specimens from patients with acute jaundice in Afghanistan (2). After passing a pooled specimen from nine subjects through a bacterial filter, he ingested the material. About 30 days later, while in Moscow, he developed jaundice, fever and abdominal pain. When he mixed his stool with convalescent sera from the patients in Afghanistan, he was able to identify clustered 27–34 mm viral particles. The virus was pathogenic for Rhesus monkeys by inoculation.
A large waterborne epidemic had occurred in Delhi, India in December, 1955–January, 1956. It was first thought to be from HAV, which had not yet been identified (3). However, after both HAV and HEV were identified, the 1955 Delhi epidemic, which affected nearly 30,000 persons, was shown to be from HEV. Numerous outbreaks with epidemiologic features similar to the Delhi outbreak had occurred regularly in the Indian subcontinent in the last several decades (4). The unique epidemiologic characteristic of these outbreaks were: (I) they involved primarily adults rather than children; (II) the epidemics did not show evidence of much secondary person to person spread; (III) the overall mortality was about 1.0%, but the mortality among pregnant women was 25% or more.
As these large waterborne outbreaks in Asia continued to occur, European countries reported patients with acute hepatitis from HEV. While many of the European cases were in immigrants or travelers from Asia or Africa, there were a growing number of autochthonous cases, who had never visited endemic areas. Phylogenetic analysis of these viruses demonstrated considerable genetic diversity. The viruses were re-classified into four subtypes 1–4, with genotypes 1 and 2 being only human pathogens and genotypes 3 and 4 having a zoonotic reservoir in swine, wild boar, deer, rabbits and shellfish (5). Recently, a new divergent strain has been isolated from a man in the United Arab Emirates and his camel and classified as genotype 7 (6).
After the new information on the zoonotic reservoir of HEV genotypes 3, 4 and 7, along with an increasing number of sporadic cases and small clusters of food borne cases of HEV in developed countries in Europe, North America and Asia, population serosurveys were done in many developed countries to estimate the prevalence and incidence of HEV infection (7). These surveys generally found quite high seroprevalence that varied considerably depending on the serologic assay that was used (Table 1). The seroprevalence in the 1988–1994 U.S. National Health and Nutrition Survey repository, representing the general population of the United States was 21.0% (8). The seroprevalence reported from European countries was similar to that in the U.S., with some countries having high prevalence, e.g., southern France, Holland, Germany (Table 1). However, the prevalence varied considerably depending on which assay was used; Wantai and Mikrogen consistently detected a substantially higher antibody prevalence than other assays (Table 1). These data suggested that some blood donors might be viremic and could transmit HEV. Several studies done to detect active infections in healthy donors reported HEV RNA prevalence varying from 1 in 1,200 in Germany, 1 in 1,438 in South of France 1 in 14,520 in Scotland (Table 2).
Table 1
| Title | Abbott (%) | Adaltis (%) | Dia.Pro (%) | Mikrogen (%) | MP (%) | Other (%) | Wantai (%) |
|---|---|---|---|---|---|---|---|
| Austria | 1.9* | 0.7* | 6.6* | 8.9* | 3.9* | 9.3* | 13.9 |
| Belgium | 4.5* | 2.5* | 10.9* | 13.8* | 7.4* | 14.3 | 19.7* |
| Czech Republic | 1.5* | 0.5* | 5.9 | 8.1* | 3.3* | 8.5 | 12.9* |
| Denmark | 4.8* | 2.8* | 11.4* | 14.3 | 7.8* | 15.2 | 19.8 |
| France | 110* | 8.7 | 21.1* | 24.7* | 16.3 | 25.4* | 31.9 |
| Germany | 2.6 | 1.1* | 7.8* | 10.3 | 4.8 | 10.8 | 29.5 |
| Italy | 0.1* | 0.1* | 2.4 | 3.9* | 0.9* | 4.1 | 7.5* |
| Netherlands | 1.8 | 0.6* | 6.4 | 8.7* | 3.7 | 9.1 | 27.0 |
| Spain | 2.2 | 0.9* | 7.1 | 9.5* | 4.3 | 10.0V | 14.7 |
| Switzerland | 1.8* | 0.6* | 4.2 | 8.8 | 4.2 | 9.2 | 21,2 |
| UK | 1.4* | 0.4* | 5.7* | 7.9* | 3.2 | 8.3* | 12.7 |
*, For combinations of seroassays and countries for which reported seroprevalence rates were not determined, the seroprevalence was calculated using a restricted maximum likelihood estimator model (R statistical platform and the metafor package)..
Table 2
| Country | Blood donors HEV RNA positive | HEV IgG Seroprevalence | Reference |
|---|---|---|---|
| Midi-Pyrenees, Southwest France •• | 1:1,438 (1:2,200) | 52.5% | Galiian |
| Germany | 1:1,200; 1:4,525 | 29.5% | Vollmerct |
| The Netherlands | 1:2,671 | 27.0% | Slot |
| England | 1:2,848; 1:7,000 | •; 12.0%; 16.0%; 16.0% | Hewitt |
| Sweden | 1:7,986 | NA | Baylis |
| Austria | 1:8,416 | 13.5% | Fischer |
| Scotland | 1:14,520 | 4.7% | Ck-land |
Seroprevalence studies have been restricted to those employing the highly-sensitive and partially-validated Wantai anti-HEV IgG assay. HEV RNA was genotype 3 in all cases. • deconstructed solvent-detergent treated mini-pools. •• Midi-Pyrénées/Méditerranées; 1:1,438, France: 1:2,200. NA, not available.
A study of blood donors in the United States found 1 in 9,000 to be HEV RNA positive (9). A study of 59,474 donations in the Netherlands used for the production of solvent/detergent treated plasma found HEV RNA in 1 of 762 donations, which is the highest rate reported in the literature (10).
Despite the high seroprevalence and the identification of HEV RNA in many healthy, otherwise qualified donors, there were many challenges to documenting transmission and estimating the risk of transfusion-transmitted HEV. The great difficulty of linkage of donors and recipients, the long incubation period of 30 days or more for infection to be manifest and the predominant risk of foodborne transmission, increased the difficulty of identifying the transmission of HEV by a transfusion. However, the risk of transfusion transmission may be significant, because most infections occur in adults, in the general population who are asymptomatic and could be acceptable blood donors.
Despite these challenges, four patients with transfusion transmitted HEV were reported in Japan in the early 2000’s; one patient was reported from Saudi Arabia (11) and one patient was diagnosed in the UK (12). Another 12 patients with transfusion transmitted HEV were detected in northern Japan, but not reported in the literature (13). Since 2005, all blood donors in the Hokkaido area have been screened routinely for HEV RNA. The investigators detected 231 HEV RNA positive donors among over 2.5 million donations (14). These donations were discarded, preventing many transfusion transmitted HEV infections in Japan.
United Kingdom study of donor HEV and transmission
The data from the largest and most comprehensive study of transfusion transmitted HEV was reported in the Lancet from southeast England in 2014 (15). In this landmark study, 225,000 blood donations between October, 2012 and September, 2013 were screened in mini-pools for HEV RNA. The study detected 79 viremic donations, which had been used to prepare 129 blood components, 62 of which had been transfused before identification of the infected donation. Follow-up of 43 recipients found 18 (42%) had evidence of HEV infection. Absence of HEV antibody and high viral load in the donation was associated with an increase in transmission. Recipient immunosuppression delayed or prevented seroconversion, prolonged the duration of viremia and increased the clinical significance of the HEV infection. Over half of the population who developed infection after their transfusion had some degree of immunocompromised and 4 patients were severely immune deficient (Table 3).
Table 3
| Patients | Primary diagnosis | Inferred immune suppression | Weeks to RNA positivity | Weeks to first detection of antibody | Duration of infection (weeks)* | Viral clearance | Alanine aminotransferase (IU/mL) | Comment |
|---|---|---|---|---|---|---|---|---|
| Patients 1–8 | ||||||||
| Patient 1 | Cardiac surgery | None | Marker not detected | 8 | NA | Yes | Not raised | No illness |
| Patient 2 | Cardiac surgery | None | Marker not detected | 14 | NA | Yes | No information | No illness |
| Patient 3 | Gastrointestinal bleeding | None | Marker not detected | 6 | NA | Yes | Not raised | No illness |
| Patient 4 | Cardiac surgery | None | 5 | 5 | 7 | Yes | 375, week 7 | Mild jaundice |
| Patient 5 | Sepsis | None | 2 | 10 | 10 | Yes | 42, week 2 | No information |
| Patient 6 | Myelodysplastic syndrome | Mild | Marker not detected | 6 | NA | Yes | Not elevated | No illness |
| Patient 7 | Myelodysplastic syndrome | Mild | Marker not detected | 3 | NA | Yes | No information | No information |
| Patient 8 | Myelodysplastic syndrome | Mild | 14 | 28 | 28 | Yes | 101, week 21 | No information |
| Median for patients 1–8 | – | – | 5 | 7 | 10 | – | – | – |
| Patients 9–14 | ||||||||
| Patient 9 | Aplastic anaemia | Moderate | 8 | Marker not detected | >12 | No† | 43, week 4 | Sepsis death† |
| Patient 10 | Metastatic cancer | Moderate | Marker not detected | 6 | NA | Yes | No information | No information |
| Patient 11 | Aplastic anaemia | Moderate | 4 | 10 | >10 | No† | 200, week 7 | Cardiac death+ |
| Patient 12 | Acute renal failure | Moderate | 3 | 11 | 11 | Yes | 148, week 9 | Steroid reduction |
| Patient 13 | Non-Hodgkin lymphoma | Moderate | 13 | 13 | >43 | No | No information | No information |
| Patient 14 | Acute myeloid leukaemia | Moderate | 12 | 21 | 25 | Yes | 1,380, week 20 | No information |
| Median for patients 9–14 | – | – | 8 | 11 | 18 | – | – | – |
| Patients 15–18 | ||||||||
| Patient 15 | Acute myeloid leukaemia | High | 17 | 38 | >40 | No | Not elevated | Deceased |
| Patient 16 | Acute myeloid leukaemia | High | 7 | Marker not detected | 16 | Yes | Not elevated | 11 weeks of ribavirin |
| Patient 17 | Failed transplant | High | 7 | Marker not detected | >10 | No† | 295‡, week 7 | Sepsis death† |
| Patient 18 | Multi organ transplant | High | 11 | 37 | 44 | Yes | 40, week 22 | Reduction of drug dose |
| Median for patients 15–18 | – | – | 9 | 37.5 | 30 | – | – | – |
Data are number, unless otherwise indicated. Median values are calculated from the numerate values in the table. *, Period from transfusion to last detection of hepatitis E virus RNA; marked > when still viraemic after the end of follow-up; †, recipient died during follow-up, so relevant data excluded from numerical analysis; ‡, transaminations thought to be secondary to abdominal sepsis and haematoma. NA, not applicable. Reprinted with permission from: Tedder RS, Ijaz S, Kitchen A, et al. Hepatitis E risks: pigs or blood-that is the question. Transfusion 2017;57:267-72.
Among the 18 infected recipients, 12 were viremic and 6 had only an antibody increase to HEV on follow-up (Table 3). HEV antibody was present in 4 (22%) of 18 donations associated with transmission and 13 (52%) of 25 donations not associated with transmission. Eight patients were immune competent and all except one of them were asymptomatic. The patient with symptoms became jaundice and had an alanine amino transferase level of 375 at week 7 after transfusion but recovered quickly. Six patients had moderate immune compromise and developed symptomatic and persistent infection. Four patients were more severely immune compromised and developed chronic or severe infection with delayed antibody response (Table 3). The data from this study, when projected across the entire country, with an estimated 8 weeks of viremia among viremic donors suggest that 80,000 to 100,000 incident HEV infections occur each year in the UK. This is similar to the 62,000 annual incidence estimated from data from two population seroprevalence studies in the UK in 1991 and 2004 (16).
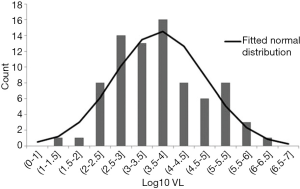
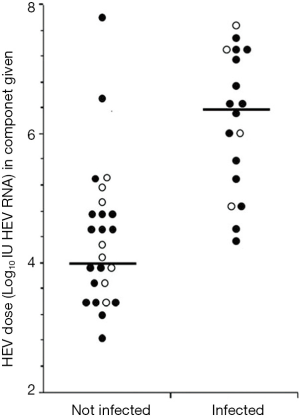
In a subsequent paper, the UK investigators estimated the volume of blood components required to reach the minimum infectious dose of 2×104 IU (17). They also estimated the ratio of foodborne to transfusion transmission in the UK considering the incidence of food borne infection of 0.2% per year (17). The distribution of HEV RNA IU level in the 79 infected donors had a log-normal distribution (Figure 1). The HEV dose in subjects who were not infected, was significantly lower than in those in whom infection occurred, however the distributions overlapped (Figure 2). The infection risk by transfusion is less that the annual dietary risk until 13 components have been transfused (17). However, some patients require larger numbers of transfused components per year, e.g., stem cell recipients, hemoglobinopathy patients and others. These patients, along with immunocompromised patients, including patients with solid organ transplants, hematologic malignancies, underlying chronic liver disease or pregnancy are at higher risk of chronic or fulminant hepatitis and should constitute a priority to receive screened HEV RNA negative blood components.
Japanese data
In addition to the data from the UK, substantial numbers of HEV exposures and infections were reported from Japan. Overall 20 transfusion transmitted HEV infections were identified in Japan from physician reports or look back. All donations had ALT levels below 60 U/L because all donors were screened and those with elevated ALT were excluded. Fifteen of nineteen transfusion transmitted HEV had elevated or indeterminate anti-IgM titers (13). Eight of 19 patients were severely immune suppressed. The total viral load in transfusion transmitted cases ranged from 3.6×104 and 1.1×108. Four patients had bimodal ALT elevation after their infection. Transmission occurred in one case despite the presence of anti HEV IgG in the donor.
Prevention
HEV is a global pathogen. Although the level of endemicity varies considerably between countries, serological or virological, evidence of HEV has been found in every country where it has been sought. In fact, nearly every country has serological evidence of infection in at least 3–5% of the general population and the seroprevalence is much higher in most countries where surveys have been done. Therefore, implementing a strategy to prevent infection of highly immunosuppressed patients by blood transfusion, as well as by dietary exposures should be considered by every country.
Although testing donors for HEV RNA and deferring positive donors is the most effective screening methods; other methods of prevention have been utilized as well. Screening of donors for elevated ALT levels was implemented prior to the discovery of hepatitis C virus (HCV) in many countries. After HCV was identified and serological and NAT assays were implemented for donor screening, ALT testing of donors was discontinued in most Western countries. However, ALT testing was continued in China and several other countries, in part because HCV NAT testing was not introduced. In a study of 9,069 qualified blood donors from four blood banks in China, those with elevated ALT had significantly greater anti-HEV IgG levels, e.g., 33.3% vs. 24.9%, and HEV antigen 1.23% vs. 0.17% than donors whose ALT was not elevated (18).
More specific markers of recent HEV infection include anti-HEV IgM and HEV antigen. In the study described, above 4 of 6 HEV RNA positive donors were HEV antigen positive. In another study of 10,741 qualified blood donors in China, 4 of 8 HEV RNA positive donors were also HEV antigen positive, whereas none of 131 anti-HEV IgM positive donors were HEV RNA positive (19). So screening donors for HEV antigen might be a simpler and cheaper method to identify a portion of infectious donors.
The preferred method for identifying HEV infectious donors is with PCR testing for HEV RNA. The data from China and Japan suggest that a viral load of about 2.0×104 is required to transmit infection. Although more data are needed on the minimal infectious dose for transfusion transmission, these data would support the use of a screening protocol using a mini pool of up to 100 donors or possibly larger.
A cost-effectiveness analysis from the Netherlands found that routine screening of donors in that country in pools of 24 would prevent most transmissions at a cost of about 300,000 euros per prevented case (20). Selective screening of donations to be transfused into immunocompromised patients would be 85% cheaper. In the Netherlands, one of 700 infections is estimated to be transfusion transmitted. However, among patients with chronic infections, one in 3.5 is estimated to be acquired by transfusion (20).
Selective NAT screening of donations for HEV RNA has been implemented in England since mid-2016. However, infections from dietary exposure are more common. Only when patients are transfused with 13 unscreened blood components would the transfusion risk equal the dietary risk in England (17). Several other European countries currently are considering implementing complete or selective screening programs at present (21).
Hopefully the risk of transfusion transmission of HEV can be minimized with wider recognition of the risk and screening of blood donations. However, endemic HEV is very likely to remain a public health concern for the foreseeable future due to continued foodborne transmissions from the global porcine reservoir, as well as other sources.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2017.09.02). KEN serves as an unpaid editorial board member of Annals of Blood from Dec 2016 to Dec 2018. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Feinstone SM, Kapikian AZ, Purceli RH. Hepatitis A: detection by immune electron microscopy of a viruslike antigen associated with acute illness. Science 1973;182:1026-8. [Crossref] [PubMed]
- Balayan MS, Andjaparidze AG, Savinskaya SS, et al. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology 1983;20:23-31. [Crossref] [PubMed]
- Viswanathan R. Infectious hepatitis in Delhi (1955-56): a critical study-epidemiology. 1957. Natl Med J India 2013;26:362-77. [PubMed]
- Labrique AB, Thomas DL, Stoszek SK, et al. Hepatitis E: an emerging infectious disease. Epidemiol Rev 1999;21:162-79. [Crossref] [PubMed]
- Hoofnagle JH, Nelson KE, Purcell RH, Hepatitis E. N Engl J Med 2012;367:1237-44. [Crossref] [PubMed]
- Woo PC, Lau SK, Teng JL, et al. New hepatitis E virus genotype in camels, the Middle East. Emerg Infect Dis 2014;20:1044-8. [Crossref] [PubMed]
- Nelson KE, Kmush B, Labrique AB. The epidemiology of hepatitis E virus infections in developed countries and among immunocompromised patients. Expert Rev Anti Infect Ther 2011;9:1133-48. [Crossref] [PubMed]
- Kuniholm MH, Purcell RH, McQuillan GM, et al. Epidemiology of hepatitis E virus in the United States: results from the Third National Health and Nutrition Examination Survey, 1988-1994. J Infect Dis 2009;200:48-56. [Crossref] [PubMed]
- Stramer SL, Moritz ED, Foster GA, et al. Hepatitis E virus: seroprevalence and frequency of viral RNA detection among US blood donors. Transfusion 2016;56:481-8. [Crossref] [PubMed]
- Slot E, Hogema BM, Riezebos-Brilman A, et al. Silent hepatitis E virus infection in Dutch blood donors, 2011 to 2012. Euro Surveill 2013;18:20550 [Crossref] [PubMed]
- Khuroo MS, Kamili S, Yattoo GN. Hepatitis E virus infection may be transmitted through blood transfusions in an endemic area. J Gastroenterol Hepatol 2004;19:778-84. [Crossref] [PubMed]
- Boxall E, Herborn A, Kochethu G, et al. Transfusion-transmitted hepatitis E in a 'nonhyperendemic' country. Transfus Med 2006;16:79-83. [Crossref] [PubMed]
- Satake M, Matsubayashi K, Hoshi Y, et al. Unique clinical courses of transfusion-transmitted hepatitis E in patients with immunosuppression. Transfusion 2017;57:280-8. [Crossref] [PubMed]
- Matsubayashi K, Sakata H, Ikeda H, et al. Hepatitis E virus infection and blood transfusion in Japan. ISBT Sci Ser 2011;6:344-9. [Crossref]
- Hewitt PE, Ijaz S, Brailsford SR, et al. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet 2014;384:1766-73. [Crossref] [PubMed]
- Ijaz S, Uyse AJ, Morgan D, et al. Indigenous hepatitis E virus infection in England: more common than it seems. J Clin Virol 2009;44:272-6. [Crossref] [PubMed]
- Tedder RS, Ijaz S, Kitchen A, et al. Hepatitis E risks: pigs or blood-that is the question. Transfusion 2017;57:267-72. [Crossref] [PubMed]
- Wang M, He M, Wu B, et al. The association of elevated alanine aminotransferase levels with hepatitis E virus infections among blood donors in China. Transfusion 2017;57:273-9. [Crossref] [PubMed]
- Ren F, Zhao C, Wang L, et al. Hepatitis E virus seroprevalence and molecular study among blood donors in China. Transfusion 2014;54:910-7. [Crossref] [PubMed]
- de Vos AS, Janssen MP, Zaaijer HL, et al. Cost-effectiveness of the screening of blood donations for hepatitis E virus in the Netherlands. Transfusion 2017;57:258-66. [Crossref] [PubMed]
- Domanovic D, Tedder R, Blumet J, et al. Hepatitis E and blood donation safety in selected European countries: a shift to screening? Euro Surveill 2017;22:30514. [Crossref] [PubMed]
Cite this article as: Nelson KE. Transfusion transmission of hepatitis E virus: an emerging issue. Ann Blood 2017;2:16.


