Chronic and occult hepatitis B virus infections in the vaccinated Chinese population
Introduction
In China, approximately 120 million people are hepatitis B virus (HBV) carriers, accounting for nearly one-third of the people infected with HBV worldwide (1). Approximately 60% of the Chinese population has been in contact with HBV. However, prevalence of hepatitis B surface antigen (HBsAg) in the Chinese population aged 1–59 years has declined from 9.5% in 1992 to 7.2% in 2006, in particular to only 1.0% of children <5 years in relation with universal hepatitis B vaccination program at birth implemented in China since 1992 (2,3).
Screening for HBsAg in collected blood units massively decreased the risk of blood-related HBV transmission, but could not detect the pre-seroconversion window period (WP) or occult HBV infection (OBI) (4). Anti-HBc screening that could largely eliminate OBIs but not WP infections (5), is not suitable for screening blood donations in China as it would reduce the blood supply by over 50% (6). The availability of nucleic acid testing (NAT) for detection of HBV DNA in blood donations enabled the identification of HBV DNA positive but HBsAg negative (HBV DNA+/HBsAg−) carriers (7). However, there are a small number of individuals with low-level viral DNA who could not be identified even by individual donation HBV NAT due to insufficient sensitivity (8). In China, the NAT of blood donations as a mandatory testing for HBV DNA and HCV and HIV RNAs has been progressively implemented nationwide for improving blood transfusion safety.
HBV universal vaccination program of infants started in 1992 in China. The population of vaccinated blood donor has been increasing since 2010, positively impacting HBV blood safety although little data is available to evidence this assumption. HBV infection, mostly OBIs with genotype B or C have been identified in 18–21 years old vaccinated individuals who received HBV vaccine derived from genotype A2 recombinant protein (9). A previous report on HBV DNA blood donors from the American Red-Cross blood services suggested that genotype A2 HBV vaccine might not be fully protective of individuals sexually exposed to highly viremic non-A2 HBV strains (10). This article intends to discuss the occurrence of HBV breakthrough and occult infections and the potential causes of HBV vaccine failure in vaccinated Chinese populations.
HBV vaccines and vaccination programs
Since the discovery of HBV in the late 1960s, 50 years have elapsed (11). The first-generation vaccines were plasma-derived HBsAg, which were initially developed in France in 1981 and in the USA in 1982 (12,13). These vaccines are still produced and used in some Asian countries. The safety of these plasma-derived vaccines was a matter of concern particularly in connection with HIV infection risk and second-generation recombinant DNA hepatitis B vaccines were produced by expressing the envelope S protein of HBV genotype A2 in yeasts (i.e., Saccharomyces cerevisiae) (12,14). These vaccines were commercialized in 1986, and were used in the worldwide, in particular as part of the routine immunization programs for infants and children or adolescents. In contrast to yeasts, the third-generation hepatitis B vaccines were developed during the 1990s in mammalian cells, including a Pre-S2/S vaccine developed in France (15), and two Pre-S1/Pre-S2/S vaccines developed in Israel (16) and Germany (17). These cell-derived Pre-S/S vaccines have already been introduced in France, Israel, and several countries of East Asia (18,19), where they appeared highly immunogenic, especially when compared to yeast-derived S protein vaccine non-responders.
The world’s first HBV universal vaccination program for infants was launched in Taiwan in July 1984 (20,21). All infants received plasma-derived HBV vaccines according to a mandatory schedule. The infants born to hepatitis B e antigen (HBeAg) positive mothers additionally received hepatitis B immunoglobulin (HBIG) 24 h after birth (22). In China, HBV vaccine was available in 1982 and the domestically produced hepatitis B vaccines were introduced on the market in 1985. The first pilot study of the universal HBV vaccination program started in 1986 in Long An county, Guangxi province (23-25). In 1992, China initiated universal hepatitis B vaccination of infants with yeast-derived vaccines (26), and hepatitis B vaccination was integrated into the national expanded program of immunization (EPI) in 2002 (27). A free nationwide catchup vaccination program was implemented in 2007 for unvaccinated children and adolescents aged 1–19 years (28,29). Since 2010 Hepatitis B vaccination was recommended to six high-risk populations, including health care workers, intravenous drug users, persons who are closely in contact with HBsAg-positive carriers, persons with high-risk sexual behavior, transfusion recipients, and hemodialysis patients (29).
In China, plasma-derived hepatitis B vaccines (5 mg/dose) were used for the first 10 years (from 1986 to 1996), and then were replaced by recombinant yeast-derived vaccine (5 mg/dose) (25). A three-dose vaccination program (0-1-6) was adopted with the first injection at any given time, and the other two at first and sixth months after the initial dose. Routine immunization was administered to all infants within 24 h of birth and subsequent doses at 1 and 6 months, respectively (2,26,28). According to the data obtained from the Global Alliance on Vaccine and Immunization project (GAVI) in China, the compliance rate of universal infant vaccination with the timely at-birth dose (TBD) increased from 20% to 91%, and the completion coverage of three-dose series of hepatitis B vaccines (HepB3) increased from 30% to 95% between 1992 and 2009 (Figure 1) (3,30). Coverage variations were found between different regions of China during two periods of 1992–2002 and 2003–2009 (Table 1) (3), in which the coverage for both TBD and HepB3 were relatively lower in the western part of China compared to those in the middle and eastern areas (30-32).
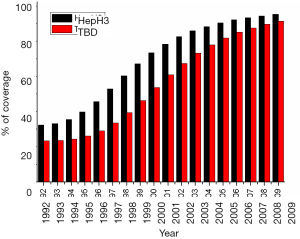
Table 1
| Region and year | Birth cohort (million) | Vaccine coverage (%) | |
|---|---|---|---|
| HepB3 | TBD | ||
| Western | |||
| 1992–2002 | 65.4 | 17–68 | 14–49 |
| 2003–2009 | 31.2 | 73–89 | 57–85 |
| 1992–2009 total | 96.6 | 17–89 | 14–85 |
| Middle | |||
| 1992–2002 | 16.3 | 29–80 | 12–60 |
| 2003–2009 | 8.4 | 83–94 | 69–94 |
| 1992–2009 total | 24.8 | 29–94 | 12–94 |
Data were estimated by the GAVI China project (
Impact of vaccination on HBV prevalence in the general population
Over the past 30 years, the effective implementation of vaccination has resulted in a substantial decrease of hepatitis B chronic carriage, in disease burden, and in hepatitis B-related morbidity and mortality (21,33-36). Universal vaccination has led to a 70–90% decrease in chronic HBV carrier rates worldwide (34), a 60% reduction in the incidence of hepatocellular carcinoma (HCC) in Taiwan (37,38). The remarkable efficacy of HBV vaccination program was best demonstrated in Taiwan, where HBsAg carrier rates decreased from 8.2% to 0.9% in the populations 26–30 years old and ≤25 years born before or after the universal program was launched on July 1, 1984 (21). In other areas with high HBV prevalence, chronic HBV carriers also dropped significantly after implementation of vaccination programs during the past 2 decades. For instance, the HBsAg positivity went from 8% to 3.7% in the general population and 0.44% in teenagers in Korea (39), from >8% to 2.7% in Vietnamese children (40), and from 13.4% to 0.9% in Afragola of Southern Italy (41).
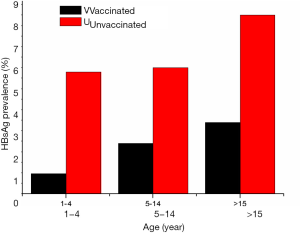
In China, approximately 2% of chronic HBV carriers reduction was observed from 9.5% to 7.2% in the general population aged 1–59 years between 1992 and 2006 since universal vaccination program was implemented nationwide (2). However, 75% to 90% reduction of HBsAg-positivity was achieved in children, whose HBsAg prevalence was reduced to 2.3% or ≤1% in children aged 5–14 or <5 years, respectively (2). HBsAg prevalence between the vaccinated and unvaccinated populations is presented in Figure 2 according to distribution in various age groups reported in 2006 (2,42). It shows the progressive decrease of chronic HBV prevalence in vaccinated Chinese population since universal infant hepatitis B immunization was launched in 1992 (43). A number of surveys also demonstrated that the great impact of hepatitis B vaccination successfully prevented the vast majority of incident chronic HBV infection and disease over the past 20 years among Chinese people in all regions across the country (3,25,29,36,44-47).
Impact of vaccination on vertical transmission of HBV from HBsAg positive mothers to infants
In Taiwan, approximately 40% of infants perinatally exposed to HBV infection from HBsAg positive but HBeAg negative (HBsAg+/HBeAg−) mothers in the pre-vaccination era without any clinical intervention became HBV chronic carriers (48). Moreover, the incidence of HBV vertical transmission was as high as 70% to 90% in infants born to mothers carrying both HBsAg and HBeAg (HBsAg+/HBeAg+) (48,49). Immunization with Hepatitis B vaccine alone or plus HBIG of the exposed newborns born to HBsAg+/HBeAg+ or HBsAg+/HBeAg− mothers could prevent 75% to 95% of chronic HBV infection (50,51). A study was carried out in 2,356 pairs of maternally exposed Taiwanese children age 6 months to 10 years between 2008 and 2009, indicating that active/passive immunization prevented 90.7% and 99.77% HBV transmission in children and overall 97.58% children were prevented from HBV vertical infection (52). Similarly, HBV vaccine efficacy was observed in a study of 1,202 pairs of Chinese infants born to HBsAg+ mothers between 2008 and 2013, which showed 100% protection for infants born to HBsAg+/HBeAg− mothers, but 90.3% protection for infants born to HBsAg+/HBeAg+ mothers whose HBV-DNA load was less than 106 copies/mL, and 96.7% vaccination efficacy in general (53).
HBV infection in the vaccinated Chinese populations
As above described, the successful introduction of universal infant hepatitis B vaccination programs had a major impact on the prevalence of HBsAg in the Chinese population. However, a small proportion of vaccinated individuals were nevertheless infected with HBV through vertical or horizontal transmission in children, adolescents and adults (2,9,21,25,54,55).
Distribution of HBV serological markers in vaccinated population
The pattern of serological markers HBsAg, anti-HBc and anti-HBs in the vaccinated population has been considerably modified since the implementation of systematic HBV vaccination program. In 1,734 new university freshmen aged around 18 years, who were vaccinated with the plasma-derived hepatitis B vaccine at birth in Taiwan, HBV markers were detected during the period September 2006 to October 2008 (54). The prevalence of serologic markers was 2.4% for HBsAg+, 5.2% (90/1734) for anti-HBc+ and 38.2% (662/1735) for anti-HBs+, respectively (Table 2). The rate of HBV-naïve subjects was 58.2%. Among HBsAg+ subjects, 1.8% were anti-HBc+ while 0.6% were anti-HBc−. Among HBsAg− subjects, 2.2% were positive for both anti-HBc+ and anti-HBs+, but 1.2% were anti-HBc only. In Qingdao, Northern China, young adults with neonatal HBV immunization were enrolled for an HBV serologic survey at age 19−21 years between 2007 and 2009 (55). Of 2,919 individuals, 2.1% were HBsAg+ and 43.9% (1281/2919) were anti-HBs+, while 14.6% (426/2919) were anti-HBc+, 4.3% of them were also anti-HBs+ but 8.3% were anti-HBc+ only. In this population, 45.8% were negative for all HBV markers. Another representative study was conducted in Shenzhen, Southern China, of 1,494 blood samples were collected between 2012 and 2013 from presumably HBV vaccinated blood donors aged 18–21 years (9). The donors were assumed to have received the systematic hepatitis B vaccines since January 1992 with the first injection at birth. The distribution of HBV serologic markers was 3.4% for HBsAg+, 65.8% (983/1494) for anti-HBs+, and 21.6% (322/1494) for anti-HBc+ including 16.5% anti-HBs+ and 1.7% anti-HBc+ only (Table 2). Approximately 31% (461/1494) of these young donors did not carry detectable anti-HBs, and 29% did not have any HBV serologic markers 18 to 21 years after vaccination. In the three regions of Taiwan, Qingdao and Shenzhen, an overall prevalence of HBsAg, anti-HBs, anti-HBc and no markers was 2.5%, 47.6%, 13.7% and 45.3%, respectively (Table 2).
Table 2
| Vaccinated population | Taiwan | Qingdao | Shenzhen | Overall |
|---|---|---|---|---|
| Age (years) | 18 | 19–21 | 18–21 | 18–21 |
| Sample collection time | 2006–2008 | 2007–2009 | 2012–2013 | 2006–2013 |
| Sample number | 1,734 | 2,919 | 1,494 | 6,147 |
| Serologic marker | ||||
| HBsAg+ [number (%)] | 41 (2.4) | 60 (2.1) | 50 (3.4) | 151 (2.5) |
| Anti-HBc+ | 30 (1.8) | 60 (2.1) | 50 (3.4) | 140 (2.3) |
| Anti-HBc− | 11 (0.6) | 0 | 0 | 11 (0.2) |
| HBsAg− [number (%)] | ||||
| Anti-HBs+/anti-HBc+ | 39 (2.2) | 124 (4.3) | 247 (16.5) | 410 (6.7) |
| Anti-HBs+/anti-HBc− | 623 (35.9) | 1,157 (39.6) | 736 (49.3) | 2,516 (40.9) |
| Anti-HBs−/anti-HBc+ | 21 (1.2) | 242 (8.3) | 25 (1.7) | 288 (4.7) |
| Anti-HBs−/anti-HBc− | 1,010 (58.2) | 1,336 (45.8) | 436 (29.2) | 2,782 (45.3) |
Data were obtained from previous studies (
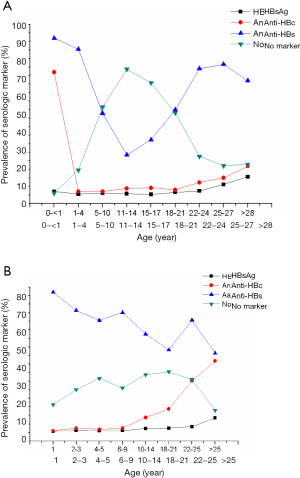
The prevalence of HBV serologic markers presented in the Taiwanese and Chinese populations according to age groups of young individuals (2,9,21,47) indicated that the prevalence of HBsAg and anti-HBc increased with age, while the frequency of vaccinees with low anti-HBs and no markers progressively declined (Figure 3A,B). The anti-HBs rate declined to the low level in the vaccinated individuals between ages 11 and 17 years, but the anti-HBs prevalence rebounded after age 18 in likely relation with boosting after HBV new contact suggested by the occurrence of HBsAg and anti-HBc positivity, followed by secondary decrease after age 25.
Breakthrough HBV infection in vaccinated individuals
In circumstances of universal infant vaccination, approximately 3% infants were vertically infected by HBsAg positive mothers, more frequently by HBeAg positive and high HBV-DNA load mothers (53,56). Among 432 HBsAg+/HBeAg+ mothers, 9.3% (40/432) infants presented with vertically transmitted HBV breakthrough infection, whose mothers all carried serum HBV-DNA load ≥6 log10 copies/mL (53). For the infants born to mothers with HBV DNA loads <6, 6–6.99, 7–7.99 or ≥8 log10 copies/mL, the infant vertical infection rates were 0%, 3.2% (3/95), 6.7% (19/282) and 7.6% (5/66), respectively (P<0.001) (56).
HBV mutations occur frequently under immune pressure, which lead to HBV mutant breakthrough infection in vaccinated children and adults by escaping from the protective immunity provided by vaccination (57-59). Among vaccinated individuals with breakthrough HBV infection, sG145R and sT126A/S mutations are prominent and account for 48% of the detected mutants in Taiwan (57). Several B- and T-cell epitopes related to HBV mutants, such as sS45T/A, sN131T, sI194V and sS207N, were detected in vaccinated children, that might escape from immune response and cause HBV infection (58). In China, the mutations in the large HBV surface protein (LHBs) sequences of HBV variants were analyzed between 1992 and 2005 to estimate the impact of universal HBV vaccination program on circulating HBV strains after 13 years (59). A total of 116 children and 112 adults of HBsAg+ samples were collected from the 2005 national survey. For comparison with HBV variants before universal immunization was initiated, samples from 157 children and 78 adults HBsAg+ were also collected from the 1992 national survey prior to mandatory vaccination. The prevalence of LHBs mutants was compared between the 1992 and 2005 surveys in child and adult populations. The prevalence of mutations in the S region of HBV mutants increased from 6.5% to 14.8% in vaccinated children, as well as significantly increasing in immunized adults after implementation of the universal infant HBV vaccination program nationwide. The G145R mutant occurred most frequently, but the frequency of this mutation was not significantly different between the vaccinated and non-vaccinated populations.
OBI in vaccinated population
Beyond breakthrough infection, a small proportion of individuals from the vaccinated population have been found HBV DNA positive but HBsAg negative (HBV DNA+/HBsAg−), meeting the definition of OBI (60). A few publications reported the prevalence of OBIs in HBV vaccinated children and adults in the Chinese population (9,55,61-63). In one such study, 186 HBV vaccinated infants born to HBsAg-positive mothers in northwestern China were examined for serologic markers and HBV DNA. Among them, 1.6% (3/186) infants were HBsAg positive, and 4.9% (9/183) infants with HBsAg-negative were identified as OBI (62). Six of nine OBI infants were positive for anti-HBs <100 IU/L and only one OBI infant was positive for anti-HBc with HBV DNA load of 3×107 IU/mL, who was born to an HBeAg positive mother with 4.0×108 IU/mL viral load. Three OBI strains were genotype C, and four recombinants genotype C/D contain an escape mutation sS143L. A recent publication reported a population-based study of children and adolescents in Taiwan. The occurrence of OBI was analyzed for the impact of universal HBV infant immunization between vaccinated and un-vaccinated cohorts (63). Among anti-HBc negative subjects, the frequency of OBI was lower in the vaccinated than in the unvaccinated cohort (0/392 vs. 4/218, P=0.007), while the frequency of OBI was higher in vaccinated than unvaccinated anti-HBc positive subjects [16/334 (4.8%) vs. 3/181 (1.7%)] although the difference was not statistically significant (P=0.072). Among both anti-HBs and anti-HBc positive subjects, OBI frequency was higher in the vaccinated than in the unvaccinated groups [13/233 (5.6%) vs. 3/170 (1.8%), P=0.025]. In the vaccinated cohort, OBI frequency was higher in anti-HBc-positive subjects than in anti-HBc-negative subjects (16/334 vs. 0/392, P<0.001). OBI carriers had lower viral load (P<0.001) and a higher mutation rate in the S region than HBsAg positive subjects. This study concluded that breakthrough HBV infections in vaccinated subjects might be associated with increased frequency of OBI compared to natural infections in unvaccinated subjects.
A total of 2,028 blood donors aged 18–25 years who had been systematically vaccinated at birth were recruited in a study at Shenzhen blood center in Southern China conducted between 2012 and 2013 (9). Twenty-four blood samples from anti-HBc+ donors were identified carrying HBV DNA+/HBsAg− with low viral load (25±22 IU/mL), in which 93.3% (14/15) strains were genotype B and 6.7% (1/15) genotype C. The follow-up of those 24 donors allowed to identify four recent infections, 17 OBIs and 3 primary OBIs. Seventy-five percent (18/24) HBV DNA+/HBsAg− donors carried anti-HBs, while half of them (9 donors) had an anti-HBs level below 100 IU/L, 8 donors between 890 and 200 IU/L, and only 1 donor over 1,000 IU/L, respectively. Among the vaccinated blood donors, the prevalence of anti-HBc, mostly associated with anti-HBs, increased from 10.7% at age 18% to 31.5% at age 25. The level of anti-HBs was significantly higher in anti-HBc positive donors than in anti-HBs only donors (P<0.0001), which suggested that recent contact with HBV boosted the anti-HBs response (9). The most likely origin of contact/infection was through sexual activity with HBsAg+ partners, although evidence of such etiology was only preliminary (9,10).
Potential causes of HBV infection in vaccinated population
None or low immune responders to HBV vaccine
In the above-described hepatitis B in 18–21 years old vaccinated Chinese population, approximately 45% of vaccinees did not carry HBV sero-markers due to the relatively rapid decline of vaccine-related antibodies (Table 2). Anti-HBs levels had been associated with protective efficacy in a vaccinated Gambian population, indicating that non-responders (anti-HBs <10 IU/L) remained susceptible to HBV and low-responders (anti-HBs <100 IU/L) were at high risk of HBV breakthrough or chronic infection (64,65). Previous vaccine trials showed up to 99% seroprotection rate in children or female adolescents (34,66). However, in adults 3–7% remained non-responders unprotected by anti-HBs <10 IU/L and 30% were low-responders 4 weeks after the last dose of a yeast-derived vaccine injection (66,67). In Shenzhen, Southern China, about 29% donors had no HBV markers who might include a small portion of non-responders (67) and a majority of vaccinees who had lost detectable anti-HBs while 40% low-responders were found in 1,494 presumably vaccinated blood donors aged 18–21 years (9). Those vaccinees with anti-HBs <100 IU/L might be vertically or horizontally associated with breakthrough or OBI as described previously (9,53,59,63). In a different study, American students with an anti-HBs level of 1 to 9 IU/L were more likely to respond to the challenge dose than those with a baseline level of 0 IU/L, which suggested that a long-term immune memory is sustained even in the absence of any detectable anti-HBs (68).
HBV genotype A2 vaccine efficacy
Current hepatitis B vaccines are derived from HBV genotype A2 clones expressing the S protein in yeast. Evidence was obtained by a study from the American red-cross that 6 of 9 HBsAg negative HBV DNA positive blood donors ranging in age between 17 and 44 years had been vaccinated (10). Five of these donors had anti-HBs <100 IU/L and had been infected with non-A2 (genotype B, C, F or D) or mixed HBV strains, while one anti-HBs negative donor was infected with HBV genotype A2 strain. This data suggested that the immunoprophylaxis efficacy of genotype A2 hepatitis B vaccine was not fully protective for individuals exposed to non-A2 strains such as genotype B, C or D.
Sexual contact with partner who carry high HBV load
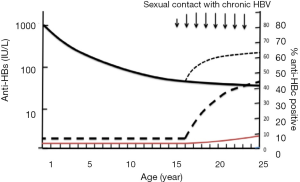
Besides vertical transmission from mothers carrying both HBsAg and HBeAg, HBV infection occurs in some vaccinated persons mostly with anti-HBs <100 IU/L or undetectable through sexual contacts with infected partners carrying high viral load HBV. It is likely that these infections are sub-clinical and do not lead to chronic infection (9,10,46,69). In those subjects, the most frequently circulating HBV strains are genotypes B, C, D or E but not A2. Persons infected with genotype C or B tend to maintain high viral load long-term correlating with high risk of sexual or vertical transmission (10,53,56), but only 20% of genotype E infected adults keep a viral load >104 (70). The evidence was further supported by the above described vaccinated American blood donors who were infected by their sexual partners carrying non-A2 HBV DNA load >1.8×106 (10). In the vaccinated Chinese blood donor population aged 18–25 years from Shenzhen blood center, approximately 60% of them were either seronegative or carrying anti-HBs <100 IU/L (9,71). Among vaccinated blood donors, the increasing proportion of anti-HBc with age is consistent with an increasing cumulative risk of HBV exposure through sexual activity (9,71), suggesting that those with low level immune response were poorly protected from contact with high HBV DNA load (10,72). To summarize the reported data, Figure 4 was constructed, which suggests that, in the vaccinated population, a small number of individuals become OBI carriers, mostly from highly infectious mothers (9,55,62,63). Later on, while they grow in age, anti-HBs levels decline to either undetectable or below 100 IU/L. When those vaccinees reaching low protective immunity are exposed to high viral load from HBV chronic carriers either vertically or sexually, an anamnestic response of anti-HBs is triggered, together with the occurrence of HBV DNA, occasionally HBsAg and the development of anti-HBc in 3–4% of cases in the vaccinated Chinese population.
Conclusions
The universal infant hepatitis B vaccination program developed in China has successfully prevented more than 90% HBV chronic infection. However, the anti-HBs <10 IU/L of non-responders and the decline of anti-HBs of vaccinees to levels below 100 IU/L appear at risk of breakthrough or OBIs. Among the vaccinated Chinese population aged between 18 and 25 years approximately 3–4% become HBV DNA carriers, after viral exposure through either vertical or horizontal or sexual contact with high viral load HBV. A new general vaccine developed from genotype B or C strains might be helpful to further reduce HBV prevalence in China in accordance with the limited data currently available in the literature. From a transfusion point of view, as previously suggested, an HBV vaccine boost between age 15 and 17 years (prior to starting sexual activity) might prevent non-clinical infections that may or may not be infectious by transfusion. Boosting at age 18–24 years would trigger an anamnestic response reaching >100 IU/L sero-protective anti-HBs levels in young adults such as college students (28,68,72). The implementation of a systematic free hepatitis B boosting vaccination program for non- and low-responders of children and adolescents can be recommended in China, which might be cost-saving and providing health benefits for the Chinese people.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No: 81371801 and 81372443), the Guangzhou Key Laboratory for Blood Safety (No. 201509010009) and the Guangzhou Pearl River S&T Nova Program (No. 201506010075).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2017.04.02). YF serves as the Editor-in-Chief of Annals of Blood. JPA serves as an unpaid editorial board member of Annals of Blood from Dec 2016 to Dec 2018. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu J, Fan D. Hepatitis B in China. Lancet 2007;369:1582-3. [Crossref] [PubMed]
- Liang X, Bi S, Yang W, et al. Epidemiological serosurvey of hepatitis B in China--declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009;27:6550-7. [Crossref] [PubMed]
- Hadler SC, Fuqiang C, Averhoff F, et al. The impact of hepatitis B vaccine in China and in the China GAVI Project. Vaccine 2013;31:J66-72. [Crossref] [PubMed]
- Biswas R, Tabor E, Hsia CC, et al. Comparative sensitivity of HBV NATs and HBsAg assays for detection of acute HBV infection. Transfusion 2003;43:788-98. [Crossref] [PubMed]
- Kleinman SH, Kuhns MC, Todd DS, et al. Frequency of HBV DNA detection in US blood donors testing positive for the presence of anti-HBc: implications for transfusion transmission and donor screening. Transfusion 2003;43:696-704. [Crossref] [PubMed]
- Zheng X, Ye X, Zhang L, et al. Characterization of occult hepatitis B virus infection from blood donors in China. J Clin Microbiol 2011;49:1730-7. [Crossref] [PubMed]
- Allain JP, Cox L. Challenges in hepatitis B detection among blood donors. Curr Opin Hematol 2011;18:461-6. [Crossref] [PubMed]
- Spreafico M, Berzuini A, Foglieni B, et al. Poor efficacy of nucleic acid testing in identifying occult HBV infection and consequences for safety of blood supply in Italy. J Hepatol 2015;63:1068-76. [Crossref] [PubMed]
- Zheng X, Ye X, Du P, et al. High prevalence of anti-hepatitis B core antigen in hepatitis B virus-vaccinated Chinese blood donors suggests insufficient protection but little threat to the blood supply. Transfusion 2015;55:890-7. [Crossref] [PubMed]
- Stramer SL, Wend U, Candotti D, et al. Nucleic acid testing to detect HBV infection in blood donors. N Engl J Med 2011;364:236-47. [Crossref] [PubMed]
- Blumberg BS, Alter HJ, Visnich S A. “new” antigen in leukemia sera. JAMA 1965;191:541-6. [Crossref] [PubMed]
- Szmuness W, Stevens CE, Harley EJ, et al. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med 1980;303:833-41. [Crossref] [PubMed]
- Plotkin SA, Orenstein WA. editors. Vaccine 2004. Philadelphia: Saunders, 2004:(299-337).
- McAleer WJ, Buynak EB, Maigetter RZ, et al. Human hepatitis B vaccine from recombinant yeast. Nature 1984;307:178-80. [Crossref] [PubMed]
- Soulié JC, Devillier P, Santarelli J, et al. Immunogenicity and safety in newborns of a new recombinant hepatitis B vaccine containing the S and pre-S2 antigens. Vaccine 1991;9:545-8. [Crossref] [PubMed]
- Shouval D, Ilan Y, Adler R, et al. Improved immunogenicity in mice of a mammalian cell-derived recombinant hepatitis B vaccine containing pre-S1 and pre-S2 antigens as compared with conventional yeast-derived vaccines. Vaccine 1994;12:1453-9. [Crossref] [PubMed]
- Wagner D, Wagenbreth I, Stachan-Kunstyr R, et al. Hepatitis B vaccination of immunosuppressed heart transplant recipients with the vaccine Hepa Gene 3 containing pre-S1, pre-S2, and S gene products. Clin Investig 1994;72:350-2. [Crossref] [PubMed]
- Shouval D. Hepatitis B vaccines. J Hepatol 2003;39:S70-6. [Crossref] [PubMed]
- Shapira MY, Zeira E, Adler R, et al. Rapid seroprotection against hepatitis B following the first dose of a Pre-S1/Pre-S2/S vaccine. J Hepatol 2001;34:123-7. [Crossref] [PubMed]
- Chen DS, Hsu NH, Sung JL, et al. A mass vaccination program in Taiwan against hepatitis B virus infection in infants of hepatitis B surface antigen-carrier mothers. JAMA 1987;257:2597-603. [Crossref] [PubMed]
- Ni YH, Chang MH, Wu JF, et al. Minimization of hepatitis B infection by a 25-year universal vaccination program. J Hepatol 2012;57:730-5. [Crossref] [PubMed]
- Hsu HM, Chen DS, Chuang CH, et al. Efficacy of a mass hepatitis B vaccination program in Taiwan. Studies on 3464 infants of hepatitis B surface antigen-carrier mothers. JAMA 1988;260:2231-5. [Crossref] [PubMed]
- Xu ZY, Duan SC, Margolis HS, et al. Long-term efficacy of active postexposure immunization of infants for prevention of hepatitis B virus infection. United States-People's Republic of China Study Group on Hepatitis B. J Infect Dis 1995;171:54-60. [Crossref] [PubMed]
- Liao SS, Li RC, Li H, et al. Long-term efficacy of plasma-derived hepatitis B vaccine among Chinese children: a 12-year follow-up study. World J Gastroenterol 1999;5:165-6. [Crossref] [PubMed]
- Shen LP, Zhang Y, Wang F, et al. Epidemiological changes in hepatitis B prevalence in an entire population after 20 years of the universal HBV vaccination programme. Epidemiol Infect 2011;139:1159-65. [Crossref] [PubMed]
- Centers for Disease Control and Prevention (CDC). Progress in hepatitis B prevention through universal infant vaccination--China, 1997-2006. MMWR Morb Mortal Wkly Rep 2007;56:441-5. [PubMed]
- Liang XF, Chen YS, Wang XJ, et al. A study on the sero-epidemiology of hepatitis B in Chinese population aged over 3-years old. Zhonghua Liu Xing Bing Xue Za Zhi 2005;26:655-8. [PubMed]
- Hutton DW, So SK, Brandeau ML. Cost-effectiveness of nationwide hepatitis B catch-up vaccination among children and adolescents in China. Hepatology 2010;51:405-14. [Crossref] [PubMed]
- Wang Z, Chen Y, Pan J. Trends of acute hepatitis B notification rates in eastern China from 2005 to 2013. PLoS One 2014;9:e114645 [Crossref] [PubMed]
- Cui F, Liang X, Gong X, et al. Preventing hepatitis B though universal vaccination: reduction of inequalities through the GAVI China project. Vaccine 2013;31:J29-35. [Crossref] [PubMed]
- Kane MA, Hadler SC, Lee L, et al. The inception, achievements, and implications of the China GAVI Alliance Project on Hepatitis B Immunization. Vaccine 2013;31:J15-20. [Crossref] [PubMed]
- Hutin Y, Hennessey K, Cairns L, et al. Improving hepatitis B vaccine timely birth dose coverage: lessons from five demonstration projects in China, 2005-2009. Vaccine 2013;31:J49-55. [Crossref] [PubMed]
- Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine 2008;26:6266-73. [Crossref] [PubMed]
- Gerlich WH. Prophylactic vaccination against hepatitis B: achievements, challenges and perspectives. Med Microbiol Immunol 2015;204:39-55. [Crossref] [PubMed]
- Ott JJ, Stevens GA, Groeger J, et al. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 2012;30:2212-9. [Crossref] [PubMed]
- Wang FS, Fan JG, Zhang Z, et al. The global burden of liver disease: the major impact of China. Hepatology 2014;60:2099-108. [Crossref] [PubMed]
- Chang MH, Chen TH, Hsu HM, et al. Prevention of hepatocellular carcinoma by universal vaccination against hepatitis B virus: the effect and problems. Clin Cancer Res 2005;11:7953-7. [Crossref] [PubMed]
- Chang MH, You SL, Chen CJ, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst 2009;101:1348-55. [Crossref] [PubMed]
- Park NH, Chung YH, Lee HS. Impacts of vaccination on hepatitis B viral infections in Korea over a 25-year period. Intervirology 2010;53:20-8. [Crossref] [PubMed]
- Nguyen TH, Vu MH, Nguyen VC, et al. A reduction in chronic hepatitis B virus infection prevalence among children in Vietnam demonstrates the importance of vaccination. Vaccine 2014;32:217-22. [Crossref] [PubMed]
- Da Villa G, Romanò L, Sepe A, et al. Impact of hepatitis B vaccination in a highly endemic area of south Italy and long-term duration of anti-HBs antibody in two cohorts of vaccinated individuals. Vaccine 2007;25:3133-6. [Crossref] [PubMed]
- Dai ZC, Qi GM. Seroepidemiological Survey in Chinese population (part one), 1992-1995. Sci Tech Exp 1996:39-59.
- Luo Z, Li L, Ruan B. Impact of the implementation of a vaccination strategy on hepatitis B virus infections in China over a 20-year period. Int J Infect Dis 2012;16:e82-8. [Crossref] [PubMed]
- Xiao J, Zhang J, Wu C, et al. Impact of hepatitis B vaccination among children in Guangdong Province, China. Int J Infect Dis 2012;16:e692-6. [Crossref] [PubMed]
- Wu JN, Wen XZ, Zhou Y, et al. Impact of the free-vaccine policy on timely initiation and completion of hepatitis B vaccination in Fujian, China. J Viral Hepat 2015;22:551-60. [Crossref] [PubMed]
- Zhu L, Zhai X, Zhu Y, et al. Evaluation of the impact of hepatitis B vaccination in adults in Jiangsu province, China. PLoS One 2014;9:e101501 [Crossref] [PubMed]
- Zhang L, Xu A, Yan B, et al. A significant reduction in hepatitis B virus infection among the children of Shandong Province, China: the effect of 15 years of universal infant hepatitis B vaccination. Int J Infect Dis 2010;14:e483-8. [Crossref] [PubMed]
- Stevens CE, Beasley RP, Tsui J, et al. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med 1975;292:771-4. [Crossref] [PubMed]
- Beasley RP, Trepo C, Stevens CE, et al. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol 1977;105:94-8. [Crossref] [PubMed]
- Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet 1983;2:1099-102. [Crossref] [PubMed]
- Beasley RP, Hwang LY, Stevens CE, et al. Efficacy of hepatitis B immune globulin for prevention of perinatal transmission of the hepatitis B virus carrier state: final report of a randomized double-blind, placebo-controlled trial. Hepatology 1983;3:135-41. [Crossref] [PubMed]
- Chen HL, Lin LH, Hu FC, et al. Effects of maternal screening and universal immunization to prevent mother-to-infant transmission of HBV. Gastroenterology 2012;142:773-81.e2. [Crossref] [PubMed]
- Zhang L, Gui XE, Teter C, et al. Effects of hepatitis B immunization on prevention of mother-to-infant transmission of hepatitis B virus and on the immune response of infants towards hepatitis B vaccine. Vaccine 2014;32:6091-7. [Crossref] [PubMed]
- Su FH, Bai CH, Chu FY, et al. Significance and anamnestic response in isolated hepatitis B core antibody-positive individuals 18 years after neonatal hepatitis B virus vaccination in Taiwan. Vaccine 2012;30:4034-9. [Crossref] [PubMed]
- Xu L, Wei Y, Chen T, et al. Occult HBV infection in anti-HBs-positive young adults after neonatal HB vaccination. Vaccine 2010;28:5986-92. [Crossref] [PubMed]
- Zou H, Chen Y, Duan Z, et al. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat 2012;19:e18-25. [Crossref] [PubMed]
- Chang MH. Breakthrough HBV infection in vaccinated children in Taiwan: surveillance for HBV mutants. Antivir Ther 2010;15:463-9. [Crossref] [PubMed]
- Lin YM, Jow GM, Mu SC, et al. Naturally occurring hepatitis B virus B-cell and T-cell epitope mutants in hepatitis B vaccinated children. ScientificWorldJournal 2013;13:571875
- Bian T, Yan H, Shen L, et al. Change in hepatitis B virus large surface antigen variant prevalence 13 years after implementation of a universal vaccination program in China. J Virol 2013;87:12196-206. [Crossref] [PubMed]
- Raimondo G, Allain JP, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol 2008;49:652-7. [Crossref] [PubMed]
- Mu SC, Lin YM, Jow GM, et al. Occult hepatitis B virus infection in hepatitis B vaccinated children in Taiwan. J Hepatol 2009;50:264-72. [Crossref] [PubMed]
- Su H, Zhang Y, Xu D, et al. Occult hepatitis B virus infection in anti-HBs-positive infants born to HBsAg-positive mothers in China. PLoS One 2013;8:e70768 [Crossref] [PubMed]
- Hsu HY, Chang MH, Ni YH, et al. Universal infant immunization and occult hepatitis B virus infection in children and adolescents: a population-based study. Hepatology 2015;61:1183-91. [Crossref] [PubMed]
- Jack AD, Hall AJ, Maine N, et al. What level of hepatitis B antibody is protective? J Infect Dis 1999;179:489-92. [Crossref] [PubMed]
- Whittle H, Jaffar S, Wansbrough M, et al. Observational study of vaccine efficacy 14 years after trial of hepatitis B vaccination in Gambian children. BMJ 2002;325:569. [Crossref] [PubMed]
- Coates T, Wilson R, Patrick G, et al. Hepatitis B vaccines: assessment of the seroprotective efficacy of two recombinant DNA vaccines. Clin Ther 2001;23:392-403. [Crossref] [PubMed]
- Han K, Shao X, Zheng H, et al. Revaccination of non- and low- responders after a standard three dose hepatitis B vaccine schedule. Hum Vaccin Immunother 2012;8:1845-9. [Crossref] [PubMed]
- Spradling PR, Xing J, Williams R, et al. Immunity to hepatitis B virus (HBV) infection two decades after implementation of universal infant HBV vaccination: association of detectable residual antibodies and response to a single HBV challenge dose. Clin Vaccine Immunol 2013;20:559-61. [Crossref] [PubMed]
- Mendy M, Peterson I, Hossin S, et al. Observational study of vaccine efficacy 24 years after the start of hepatitis B vaccination in two Gambian villages: no need for a booster dose. PLoS One 2013;8:e58029 [Crossref] [PubMed]
- Candotti D, Danso K, Allain JP. Maternofetal transmission of hepatitis B virus genotype E in Ghana, west Africa. J Gen Virol 2007;88:2686-95. [Crossref] [PubMed]
- Ye X, Li T, Xu X, et al. Characterisation and follow-up study of occult hepatitis B virus infection in anti-HBc-positive qualified blood donors in southern China. Blood Transfus 2016;15:6-12. [PubMed]
- Wang Z, Zeng J, Li T, et al. Prevalence of hepatitis B surface antigen (HBsAg) in a blood donor population born prior to and after implementation of universal HBV vaccination in Shenzhen, China. BMC Infect Dis 2016;16:498. [Crossref] [PubMed]
Cite this article as: Li T, Fu Y, Allain JP, Li C. Chronic and occult hepatitis B virus infections in the vaccinated Chinese population. Ann Blood 2017;2:4.