
Optimizing total nucleated cell yield in bone marrow harvests for hematopoietic stem cell transplantation
Introduction
Background
The transplantation of hematopoietic stem cells (HSCs) has become part of the routine management of malignant and non-malignant blood cell disorders, autoimmune diseases, and inborn errors of immunity and metabolism (1). HSC are collected from human leucocyte antigen (HLA) matched related or unrelated volunteer donors or less commonly from stored umbilical cord blood (2). HSC from volunteer donors is collected by bone marrow harvest (BMH) or granulocyte colony-stimulating factor mobilized peripheral blood stem cells (PBSCs) (3). While overall survival rates are similar for adult recipients of PBSC and BMH, PBSC is associated with faster hematologic recovery and a lower risk of graft failure, while carrying a higher risk for chronic graft-versus-host-disease (GvHD) due to an increased presence of T-cells in peripheral blood (4-6). BMH carries lower mortality rates and lower acute and chronic GvHD risks than PBSC in children and adolescents suffering from leukemia (7). BMH also shows lower transplant related mortality rates than PBSC in children with non-malignant blood disorders (8).
Today, 80.3% of HSC are generated by PBSC worldwide, while 10.6% come from BMH and 9% from banked cord blood (9). BMH remains an important source of HSC in non-malignant blood disorders when graft-versus-malignancy effects are not important (10), in pediatric HSC recipients (7,8) and in conditions when GvHD is a clinical concern. GvHD risks are increased for patient age >50 years, donor age >32 years, a diagnosis of chronic myeloid leukemia, a lack of anti-T cell globulin for the conditioning regimen, a high/very high Dana Farber Disease Risk Index, an unrelated donor HSC source, HLA-mismatching, or a high-parity female donor (5,11-13). Associated with the decreasing use of BMH has been a decline in the quality of the procedure as measured by the total nucleated cell (TNC) dose collected in recent years. Fewer collection centers have accumulated the necessary expertise for optimal procedure outcomes. Centers performing at least 30 BMH per year show better TNC yield than smaller programs (14).
Rationale and knowledge gap
The MedStar Georgetown University Hospital Blood and Marrow Collection Program (MGUH-BMCP) is the largest BMH center in the US with more than 250 allogeneic harvest procedures per year. In 2023, 292 allo-BMH were performed at the program which constitute 10.6% of all BMH recorded worldwide by the World Marrow Donor Association (9). The program is a dedicated partner of the National Marrow Donor Program (NMDP) and a member of the NMDP alliance of collection centers (15). This clinical review shall help collection centers and proceduralists to optimize TNC yield in allogeneic BMH based on the experience with more than 1,300 procedures conducted at MGUH-BMCP between January 2018 and June 2024. The review will discuss clinical aspects of BMH from donor selection through technical considerations of the procedure itself and post-procedure donor care emphasizing the optimization of TNC yield. The integration of donor anthropometric and laboratory data with clinical procedural experiences has not been done at this time. It can help stem cell collection centers to develop guidelines for their own clinical best practice.
Objective
Optimizing TNC yield in BMHs is a multi-faceted process. Transplantation outcomes and recipient survival depend on high quality harvest products. Best-practice guidelines and clinical reviews can help stem cell collection centers to deliver that quality.
Methods
This review discusses individual aspects of the BMH process for allogeneic donors in detail using data analysis from MGUH-BMCP donors together with the relevant current literature to support statements and recommendations. Anthropometric and hematological donor characteristics are summarized using mean and standard deviation (SD) for continuous and percentages for categorical variables (Table 1). T-testing is used to compare continuous variables after controlling for normality and testing for equal variance, while categorical variables like smoker status or the use of pre-procedural blood donation are compared by Chi-squared testing. Spearman’s correlation coefficients are computed to assess the correlation between variables. Linear and multiple regression models are fitted to evaluate the association between variables and TNC yield including the computation of R2 levels. 95% confidence intervals (CI) are determined, and P values of less than 0.05 are considered statistically significant. Stata® 18th edition software is used for the statistical analysis.
Table 1
| Donor and product characteristics | Total (n=1,375) | Male (n=775) | Female (n=600) | P value (male vs. female) |
|---|---|---|---|---|
| Donor characteristics | ||||
| Age (years) | 29.1±7.13 | 28.8±6.94 | 29.6±7.36 | 0.04* |
| Weight (kg) | 82.1±17.39 | 87.9±16.39 | 74.7±15.66 | <0.001* |
| Body mass index (kg/m2) | 27.3±4.94 | 27.3±4.60 | 27.3±5.36 | 0.94 |
| Donor recipient weight ratio | 2.7±2.85 | 2.7±2.86 | 2.7±2.84 | 0.97 |
| Smoker | 249 (18.1) | 174 (12.7) | 75 (5.5) | <0.001** |
| Hemoglobin (g/dL) | 14.3±1.40 | 15.1±1.04 | 13.2±0.99 | <0.001* |
| Platelets (109/L) | 255±57.8 | 239±50.5 | 276±60.1 | <0.001* |
| White blood cells (109/L) | 6.5±1.74 | 6.2±1.59 | 6.7±1.90 | <0.001* |
| Estimated blood volume (L) | 4.995±0.91 | 5.542±0.69 | 4.289±0.63 | <0.001* |
| Marrow product characteristics | ||||
| Marrow volume collected (mL) | 1,096±349.28 | 1,161±364.07 | 1,012±309.67 | <0.001* |
| Percent blood volume collected | 23.9±7.73 | 22.8±7.56 | 25.4±7.70 | <0.001* |
| BMH duration (min) | 38.8±17.64 | 37.8±16.47 | 40.2±18.97 | 0.01* |
| TNC/kg requested (NC × 108/kg) | 5.1±2.17 | 5±2.18 | 5.2±2.15 | 0.06 |
| TNC/kg collected (NC × 108/kg) | 7.1±5.73 | 7±5.87 | 7.2±5.54 | 0.67 |
| TNC requested (NC × 108) | 245±128.90 | 261±134.44 | 224±116.76 | <0.001* |
| TNC collected (NC × 108) | 261±85.90 | 278±86.22 | 239±80.42 | <0.001* |
| TNC yield (NC × 106/mL) | 19.3±5.73 | 19.6±5.87 | 19±5.54 | 0.10 |
Comparing male with female donors. Continuous variables were presented as means ± standard deviations and categorical variables were presented as numbers (percentages). *, indicating statistical significance in t-test analysis; **, indicating statistical significance in Chi-squared analysis. BMH, bone marrow harvest; MGUH-BMCP, MedStar Georgetown University Hospital Blood and Marrow Collection Program; TNC, total nucleated cell; NC, nucleated cell.
Results and discussion
Donor characteristics—donor age
The donor-recipient matching process depends on the compatibility of donor and recipient HLA pairs. While HLA-identical sibling donors are available to a minority of patients, 70% of recipients rely on unrelated HLA-matched donors. European transplant centers consider a 10/10 match for the HLA-A, -B, -C, -DRB1, and -DQB1 alleles their gold standard. The US-based NMDP uses an 8/8 match of HLA-A, -B, -C, and -DRB1 (16,17). Newer techniques in high-resolution HLA-typing can help to understand the effects of “non-classical” HLA types on graft failure or GvHD (18,19), while improved immunosuppressive therapeutics like anti-thymocyte globulin (20) or post-transplant cyclophosphamide allow for the transplantation of mismatched HLA alleles (21,22).
Beyond the genetic matching of HLA alleles, a variety of donor factors may affect the quality of the stem cell product and patient outcomes. Donor age appears to be inversely related to recipient survival (19,23). The grafts from younger donors contain more CD34+ cells while those of older donors have increased numbers of natural killer (NK) cells and carry more “antigen-experienced” memory T-cells, which might be responsible for chronic GvHD (12), particularly in donor ages beyond 50 years (24,25). Case studies report that patients with advanced myelodysplastic syndrome and acute lymphoblastic leukemia have better 5-year overall survival (26) and lower relapse rates (27) when they receive HSC transplants from younger unrelated donors than older HLA-identical siblings. Donor cytomegalovirus (CMV) status, ABO blood group, gender and parity are of lesser importance (28).
The donors at MGUH-BMCP are mostly young adults in their 20s and 30s (Table 1), making the study of the effects of age on TNC yield difficult. The correlation between donor age and TNC yield at the center is weakly negative (Spearman coefficient r=−0.086; P=0.001) and reflects the narrow age range of the center’s donor population.
Donor characteristics—donor size
It is well known that with increasing volume of the BMH product, cell density decreases. This is largely due to the dilution of marrow with peripheral blood during higher harvest volumes (29-31). Studies reporting a positive correlation between donor weight and body mass index (BMI) on the one side and TNC yield on the other (29,31,32) often fail to make a connection between donor size, recipient cell needs and harvest volume. Anthias et al. (33) found that BMHs generated from donors weighing less than their respective recipients were less likely to reach engraftment thresholds of 2×108 TNC/kg of recipient weight.
Using the donor-recipient weight ratio rather than donor weight or BMI (34) and the percentage of donor estimated blood volume harvested rather than total harvest volume (35) allows to connect recipient needs with donor characteristics (Figure 1).
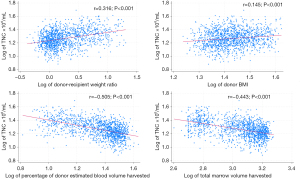
While it is important to know how donor characteristics influence TNC density per milliliter of marrow, it is more important to determine, how donor characteristics affect the goal to meet recipients’ TNC requirements. Here, the donor-recipient weight ratio is an informative variable accounting for 80% of the variance of TNC yield per kg of recipient weight (Figure 2). When the donor’s weight is less than 60% of the recipient’s, the threshold for engraftment (2×108 TNC/kg) can barely be reached in 50% of cases. Meeting the engraftment threshold can be guaranteed with 90% certainty only when the donor is heavier than the recipient (Table 2).

Table 2
| Donor-recipient weight ratio | TNC × 108/kg |
|---|---|
| ≤0.6 (n=11) | 2.04 (1.83–2.6) |
| >0.6–≤0.8 (n=85) | 2.9 (1.88–5.1) |
| >0.8–≤1 (n=181) | 3.44 (1.75–5.8) |
| >1 (n=1,096) | 6 (2–46.2) |
| Total (n=1,373) | 5.19 (1.7–46.2) |
Median TNC counts (90% confidence intervals) are displayed. TNC, total nucleated cell.
Donor characteristics—smoker status
Donors who identify as current smokers have been reported as having higher TNC counts per mL than non-smokers (35). Hematopoiesis in smokers may be stimulated by toxin effects on TET2 mutations (36), stimulation of receptors by nicotine (37), or by cytokine release of pulmonary alveolar macrophages (38). Donors identifying as current smokers at MGUH-BMCP show a slightly higher TNC yield per mL than nonsmokers (20.05×106/mL vs. 19.17×106/mL; 95% CI: 0.09, 1.66; P=0.029). This difference becomes insignificant (P=0.074) when looking at TNC yield per kg of recipient weight. We are also unable to control data for the extent and duration of donors’ smoking habits.
Donor characteristics—laboratory indicators
Matching donors with recipients is important not only with respect to their genetic compatibility but also with respect to their size. In addition, laboratory markers generated prior to the procedure can give the proceduralist indications about the cellularity of the bone marrow at the time (Figure 3). white blood cell (WBC) (r=0.363; P<0.001) and platelet count (r=0.191; P<0.001) correlate with TNC yield per mL of bone marrow with mild impact on cell yield per kg of recipient weight (WBC: r=0.164; P<0.001; platelet: r=0.101; P<0.001).

HSC-niche components like the sinusoidal endothelium, perivascular stromal cells, macrophages, megakaryocytes, osteoblasts, and sympathetic nerves interact with cytokines, growth factors and a variety of chemical influences to stimulate and regulate thrombopoiesis, granulopoiesis and hematopoiesis within the bone marrow. This can explain the correlation between WBC and platelet levels and TNC density within the marrow (39-41).
Donor hemoglobin (Hb) levels are barely associated with cell yield per mL and do not affect TNC levels per kg of recipient weight. Nevertheless, Hb levels are important to assess the potential marrow volume that can be accessed safely for harvesting. At MGUH-BMCP, female donors experience a 1-g/dL drop in their Hb for each 300 mL of bone marrow harvested irrespective of the starting Hb level, making this a simple and reliable method to predict procedural blood loss. Hb levels in male donors drop by slightly less (Figure 4). Alternatively, proceduralists can roughly calculate post-procedure hematocrit levels using the equation: [estimated blood volume × Hct − harvest volume × 0.3 (assumed bone marrow Hct) − estimated soft tissue blood loss × Hct]/estimated blood volume = post-procedure Hct (15).

In a linear regression analysis, all studied donor laboratory characteristics influence TNC/mL levels to a minor degree (between 1% for Hb and 12% for WBC). Applying a multiple regression analysis with stepwise backward elimination to the model, all characteristics retain their significance in influencing TNC/mL. The complete model explains 22.7% of the TNC/mL variance (Table 3).
Table 3
| Donor characteristics | TNC per mL | TNC per kg | |||||
|---|---|---|---|---|---|---|---|
| Marrow harvested | R2 | Recipient weight | R2 | ||||
| Correlation coefficients (95% CI) | P value | Correlation coefficients (95% CI) | P value | ||||
| Age | −0.09 (−1.30 to −0.04) | <0.001* | 0.01 | −0.04 (−0.08 to 0.00) | 0.06 | 0.003 | |
| Donor-recipient weight ratio | 0.62 (0.52 to 0.72) | <0.001* | 0.10 | 1.79 (1.74 to 1.84) | <0.001* | 0.80 | |
| BMI | 0.17 (0.11 to 0.23) | <0.001* | 0.02 | 0.03 (−0.04 to 0.09) | 0.42 | 0.001 | |
| WBC | 1.13 (0.98 to 1.30) | <0.001* | 0.12 | 0.27 (0.10 to 0.44) | 0.002* | 0.007 | |
| Platelets | 0.02 (0.01 to 0.02) | <0.001* | 0.03 | 0.005 (0.0 to 0.01) | 0.08 | 0.002 | |
| Hemoglobin | 0.29 (0.07 to 0.51) | 0.009* | 0.01 | 0.20 (−0.02 to 0.42) | 0.07 | 0.002 | |
*, indicates statistical significance on F-testing. TNC, total nucleated cell; CI, confidence interval; BMI, body mass index; WBC, white blood cell.
The regression analysis looks very different when analyzing the effects of donor characteristics on TNC/kg of recipient weight. Only the donor-recipient weight ratio and donor WBC are significantly related to the TNC/kg of recipient weight. The donor-recipient weight ratio explains 80% of the TNC/kg variance with WBC accounting for a mere 0.7% (Table 3). This underlines the importance of the donor-recipient weight ratio for providing the recipient with an adequate TNC count (34).
Procedure characteristics—pre-procedure autologous blood donation (PAD)
While many BMH centers have abandoned the collection of pre-procedural autologous blood units for post-procedural reinfusion (42,43), others continue the practice for prospective large volume bone marrow collections (44). Associated risks include bacterial contamination, the erroneous use of incompatible blood units, hemolysis, wastage of unused blood products and the incomplete Hb recovery prior to the BMH (45-48).
Comparing female and male donors with and without PAD who donated at least 1,000 mL of bone marrow at MGUH-BMCP, we notice that donors with PAD do not recover their pre-PAD Hb levels at the time of the BMH, neutralizing the potential benefits of PAD for post-harvest Hb recovery. Consequently, post-harvest Hb levels are similar for donors with and without PAD, except for a few female donors without PAD, who drop their Hb levels below a transfusion threshold of 7 g/dL (Table 4). These donors all donated more than 30% of their total estimated blood volume during the procedure. Therefore, the lowest of 30% of estimated blood volume, 18 mL/kg of donor weight, or 1,500 mL collection volume have been established at MGUH-BMCP as the volume limit for BMHs in female donors, while maintaining the lowest of 33% of estimated blood volume, 20 mL/kg, or 1,500 mL for their male counterparts.
Table 4
| Donor sex | Donor Hb | No PAD | PAD | Chi-squared | P value | |||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | n | Mean (SD) | n | |||||
| Female donors | Hb before PAD (g/dL) | 13.5 (1.03) | 270 | 13.6 (0.96) | 76 | 38.812 | 0.96 | |
| Hb before BMH (g/dL) | 13.2 (0.96) | 270 | 12.7 (0.93) | 76 | 57.047 | 0.13 | ||
| Hb after BMH (g/dL) | 9.29 (1.24) | 270 | 9.4 (0.93) | 76 | 47.928 | 0.77 | ||
| Male donors | Hb before PAD (g/dL) | 15.4 (1.04) | 410 | 15.3 (0.99) | 125 | 55.039 | 0.51 | |
| Hb before BMH (g/dL) | 15.2 (1.01) | 410 | 14.6 (1.07) | 125 | 88.446 | 0.006* | ||
| Hb after BMH (g/dL) | 11.2 (1.29) | 408 | 11.3 (1.27) | 124 | 63.255 | 0.50 | ||
*, indicates statistical significant difference on Chi-squared testing. Hb, hemoglobin; PAD, procedure autologous blood donation; BMH, bone marrow harvest; SD, standard deviation.
Procedure characteristics—single syringe aspiration volumes and aspiration needle type
The impact of individual syringe aspiration volume during BMHs has been discussed in the past. While some studies advocate the use of small 2–5 mL aspiration volumes as a strategy to maximize TNC yield (49,50), others find no significant differences between small and large aspiration volumes while emphasizing the value of fewer bone penetrations for donor recovery (51,52). Studies reporting large differences between small and large aspiration volumes used single terminal hole aspiration needles (49,50). Others emphasize that the use of multi-hole fenestrated needles allows for the aspiration of marrow from multiple directions simultaneously making the choice of aspiration volume in each syringe less relevant (53,54).
At MGUH-BMCP we find that 10 mL aspiration volumes yield a higher TNC content per mL than 20 mL aspiration volumes (27.8×106/mL vs. 25.1×106/mL; 95% CI: 1.17, 4.39; P=0.001) using multi-hole fenestrated aspiration needles. These values are controlled for procedure volume by only including bone marrow collections of 600 mL. Procedures using 10 mL aspirations last 54.7% longer than procedures using 20 mL aspiration volumes (11.82 vs. 7.64 minutes; 95% CI: 3.56, 4.80; P<0.001).
The impact of individual syringe aspiration volume may increase with larger total collection volumes, and proceduralists should consider multiple variables including donor-recipient weight ratio, percentage of donor blood volume to be harvested, procedure duration, ease of marrow flow during collection, donor WBC count, type of needle used, mid-procedure TNC count and final target TNC count when deciding on the individual syringe volumes to be employed.
Procedure characteristics—mid-procedural TNC count
Collecting a mid-procedural TNC measurement allows the proceduralist to predict the necessary final BMH volume and adjust procedural technique (15,54). For example, a lower-than-expected TNC count would invite the proceduralist to reduce single syringe aspiration volumes for the second half of the procedure in order to maximize TNC yield. Conversely, with higher-than-expected TNC counts the proceduralist may increase aspiration volumes to reduce the number of needle penetrations and limit procedure time.
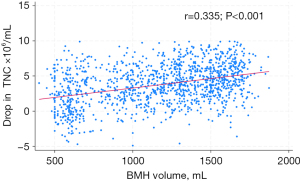
At MGUH-BMCP, we notice a linear correlation between TNC drop and collection volume (35) (Figure 5). This allows the proceduralist to estimate the remaining harvest volume necessary to meet recipient cell needs, once a mid-procedural TNC count has been established at the 600 mL collection point. For example, the final TNC yield/mL for a 1,500 mL collection would be 5×106 lower than the TNC count generated at the 600 mL collection point. We advise proceduralists to subtract 5×106/mL from the mid-procedural TNC count to estimate the necessary remaining collection volume. If the requested TNC count is 250×108 and the mid-procedural TNC yield is 25×106/mL, the final volume required is: 250/(25 − 5) × 100 = 1,250 mL.
Procedure characteristics—intraprocedural fluid replacement
The recommendations for intraoperative fluid management during BMHs include the replacement of colloid bone marrow with crystalloid fluids such as Ringer’s Lactate at a 2:1 ratio with an additional aliquot given postoperatively for a total of 3:1 fluid replacement. Alternatively, colloid fluids such as 5% albumin can be used at a 1:1 ratio to limit total fluid replacement volumes (15). Proceduralists might be tempted to reduce fluid replacement volumes assuming a negative correlation between the donor’s hydration level and TNC yield.
At MGUH-BMCP, a negative correlation between fluid replacement and TNC yield cannot be confirmed. In fact, there is a mildly positive correlation between fluid-harvest volume ratio and TNC/mL of marrow (Spearman correlation coefficient r=0.082; P=0.016) for harvests of at least 1,000mL. The coefficient for TNC yield per kg of recipient weight is equally positive (r=0.086; P=0.011) indicating that more rather than less fluid replacement contributes slightly to TNC yield. In a regression analysis, the fluid-harvest volume ratio contributes 0.6% to TNC/mL yield (R2=0.006; 95% CI: 0.09, 1.08; P=0.02) and 0.6% to TNC yield per kg of recipient weight (R2=0.006; 95% CI: 0.05, 0.57; P=0.017) for harvest volumes of at least 1,000 mL.
Donors who were able to leave the hospital on the day of the procedure had received an average of 2.0 times their marrow donation volume intraoperatively as crystalloid replacement, while donors who had to spend a night at the hospital postoperatively received 1.8 times their donation volume intraoperatively (95% CI: 0.09, 0.27; P<0.001). Similarly, donors who experienced orthostasis after the procedure received less fluid replacement than their asymptomatic counterparts (1.58 vs. 1.73 times harvest volume; 95% CI: 0.06, 0.25; P=0.002). These differences disappear when looking only at harvest volumes of at least 1,000 mL. Consequently, there is no reason to reduce intraoperative fluid replacement. Fluid restriction does not increase TNC yield while causing potential post-procedural discomfort to the donor.
Limitations
This clinical review is based on the experiences generated at a single center. Nevertheless, this center provides the majority of all BMH performed in the US. All BMH reviewed come from unrelated young adult donors, making suggested relations between donor age and TNC yield less reliable. Data on donor smoking status could not be specified for duration or intensity of smoking. The investigation of single syringe volume effects on TNC yield has been limited to small total volume collections to limit the confounding effects of total harvest volume. It may be advisable to conduct a similar investigation with larger harvest volumes. Intraprocedural fluid management at the MGUH-BMCP is based on crystalloid replacement fluids. At other centers, colloid fluids like 5% albumin solution may be a preferred management option. A comparison between different fluid replacement strategies may be of interest.
Highlights
- The donor-recipient weight ratio accounts for 80% of TNC/kg of recipient weight variance.
- Donor WBC and platelets counts correlate mildly with TNC yield.
- Donor Hb levels do not correlate with TNC yield but affect potential total harvest volume.
- Pre-PAD is not recommended.
- Limiting harvest volumes to 30% of estimated blood volume in female donors prevents the need for post-procedure transfusion.
- Smaller individual aspiration volumes can increase TNC yield but must be weighed against procedure time and number of bone penetrations.
- Mid-point TNC measurements facilitate the estimation of final TNC yield.
- Restricting intra-procedural fluid replacement does not enhance TNC yield and can be associated with post-procedural complications.
Conclusions
BMH remains a valuable tool in the HSC transplantation process for adult and pediatric recipients. With fewer centers engaging in high procedure volumes, proceduralists can benefit from strategic guidelines to maintain and improve training and skill levels. Donor characteristics beyond the HLA-matching process and technical procedure aspects affect TNC yield and eventual outcome for the HSC transplantation recipients.
When selecting the right HLA-matched donor, the donor-recipient weight ratio has the greatest impact on the eventual TNC yield per kg of recipient weight. Donor age and donor laboratory characteristics like WBC and platelet counts also have minor influence on TNC yield but much less than the donor-recipient weight ratio.
Donor Hb levels do not impact TNC yield but are important measures to assess the potential marrow volume that can be accessed safely for BMH. Preprocedural autologous blood donation has no advantage for cell yield but may affect donor care negatively. It should be abandoned as a routine measure. Rather, judicious harvest volume limitations should be employed to maintain maximal donor safety.
During the procedure, smaller individual syringe aspiration volumes are associated with higher TNC yield but must be weighed against procedure time and potential donor discomfort related to more numerous needle penetrations. Proceduralists are advised to consider the donor-recipient weight ratio, donor laboratory characteristics, mid-procedure TNC yield, ease of marrow flow, TNC goals and procedure time when deciding on a prospective single syringe aspiration volume. The mid-procedure TNC yield is a helpful measure to estimate the further necessary collection volume.
Intra-procedural fluid restriction carries no advantage for TNC yield and may compromise donor safety. Fluid replacement with crystalloid solution should aim to replace marrow volume harvested in a 3:1 fashion, using two aliquots during the procedure and a further aliquot afterwards.
Optimizing TNC yield in BMHs is a multi-faceted process. Transplantation outcomes and recipient survival depend on high quality harvest products. Best-practice guidelines and clinical reviews can help stem cell collection centers to deliver that quality.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://aob.amegroups.com/article/view/10.21037/aob-24-19/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://aob.amegroups.com/article/view/10.21037/aob-24-19/coif). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Snowden JA, Sánchez-Ortega I, Corbacioglu S, et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant 2022;57:1217-39. [Crossref] [PubMed]
- Confer DL, Miller JP, Chell JW. Bone marrow and peripheral blood cell donors and donor registries. In: Forman SJ, Negrin RS, Antin JH, et al. editors. Thomas’ Hematopoietic Cell Transplantation. Oxford: Wiley Blackwell; 2016:423-30.
- Hequet O. Hematopoietic stem and progenitor cell harvesting: technical advances and clinical utility. J Blood Med 2015;6:55-67. [Crossref] [PubMed]
- Amouzegar A, Dey BR, Spitzer TR. Peripheral Blood or Bone Marrow Stem Cells? Practical Considerations in Hematopoietic Stem Cell Transplantation. Transfus Med Rev 2019;33:43-50. [Crossref] [PubMed]
- Alousi A, Wang T, Hemmer MT, et al. Peripheral Blood versus Bone Marrow from Unrelated Donors: Bone Marrow Allografts Have Improved Long-Term Overall and Graft-versus-Host Disease-Free, Relapse-Free Survival. Biol Blood Marrow Transplant 2019;25:270-8. [Crossref] [PubMed]
- Holtick U, Albrecht M, Chemnitz JM, et al. Comparison of bone marrow versus peripheral blood allogeneic hematopoietic stem cell transplantation for hematological malignancies in adults - a systematic review and meta-analysis. Crit Rev Oncol Hematol 2015;94:179-88. [Crossref] [PubMed]
- Keesler DA, St Martin A, Bonfim C, et al. Bone Marrow versus Peripheral Blood from Unrelated Donors for Children and Adolescents with Acute Leukemia. Biol Blood Marrow Transplant 2018;24:2487-92. [Crossref] [PubMed]
- Zaucha-Prażmo A, Sadurska E, Pieczonka A, et al. Risk Factors for Transplant Outcomes in Children and Adolescents with Non-Malignant Diseases Following Allogeneic Hematopoietic Stem Cell Transplantation. Ann Transplant 2019;24:374-82. [Crossref] [PubMed]
- World Marrow Donor Association. WMDA global trend report 2023. Accessed 20 June 2024. Available online: https://wmda.info/storage/2024/06/05062024-GTR-2023-Summary-slides.pdf
- Bhella S, Majhail NS, Betcher J, et al. Choosing Wisely BMT: American Society for Blood and Marrow Transplantation and Canadian Blood and Marrow Transplant Group’s List of 5 Tests and Treatments to Question in Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2018;24:909-13. [Crossref] [PubMed]
- Solh M, Zhang X, Connor K, et al. Factors Predicting Graft-versus-Host Disease-Free, Relapse-Free Survival after Allogeneic Hematopoietic Cell Transplantation: Multivariable Analysis from a Single Center. Biol Blood Marrow Transplant 2016;22:1403-9. [Crossref] [PubMed]
- Kollman C, Spellman SR, Zhang MJ, et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood 2016;127:260-7. [Crossref] [PubMed]
- Timofeeva OA, Philogene MC, Zhang QJ. Current donor selection strategies for allogeneic hematopoietic cell transplantation. Hum Immunol 2022;83:674-86. [Crossref] [PubMed]
- Prokopishyn NL, Logan BR, Kiefer DM, et al. The Concentration of Total Nucleated Cells in Harvested Bone Marrow for Transplantation Has Decreased over Time. Biol Blood Marrow Transplant 2019;25:1325-30. [Crossref] [PubMed]
- Murakami MA, Connelly-Smith L, Spitzer T, et al. Bone Marrow Harvest: A White Paper of Best Practices by the NMDP Marrow Alliance. Transplant Cell Ther 2024;30:663-80. [Crossref] [PubMed]
- Dehn J, Spellman S, Hurley CK, et al. Selection of unrelated donors and cord blood units for hematopoietic cell transplantation: guidelines from the NMDP/CIBMTR. Blood 2019;134:924-34. [Crossref] [PubMed]
- Yu N, Askar M, Wadsworth K, et al. Current HLA testing recommendations to support HCT. Hum Immunol 2022;83:665-73. [Crossref] [PubMed]
- Neuchel C, Gowdavally S, Tsamadou C, et al. Higher risk for chronic graft-versus-host disease (GvHD) in HLA-G mismatched transplants following allogeneic hematopoietic stem cell transplantation: A retrospective study. HLA 2022;100:349-60. [Crossref] [PubMed]
- Crocchiolo R, Rombolà G. Human Leucocyte Antigen System and Selection of Unrelated Hematopoietic Stem Cell Donors: Impact of Patient-Donor (Mis)matching and New Challenges with the Current Technologies. J Clin Med 2023;12:646. [Crossref] [PubMed]
- Walker I, Panzarella T, Couban S, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol 2016;17:164-73. Erratum in: Lancet Oncol 2018;19:e581. [Crossref] [PubMed]
- Shaw BE, Jimenez-Jimenez AM, Burns LJ, et al. National Marrow Donor Program-Sponsored Multicenter, Phase II Trial of HLA-Mismatched Unrelated Donor Bone Marrow Transplantation Using Post-Transplant Cyclophosphamide. J Clin Oncol 2021;39:1971-82. [Crossref] [PubMed]
- Nunes NS, Kanakry CG. Mechanisms of Graft-versus-Host Disease Prevention by Post-transplantation Cyclophosphamide: An Evolving Understanding. Front Immunol 2019;10:2668. [Crossref] [PubMed]
- Shaw BE, Logan BR, Spellman SR, et al. Development of an Unrelated Donor Selection Score Predictive of Survival after HCT: Donor Age Matters Most. Biol Blood Marrow Transplant 2018;24:1049-56. [Crossref] [PubMed]
- Guru Murthy GS, Kim S, Hu ZH, et al. Relapse and Disease-Free Survival in Patients With Myelodysplastic Syndrome Undergoing Allogeneic Hematopoietic Cell Transplantation Using Older Matched Sibling Donors vs Younger Matched Unrelated Donors. JAMA Oncol 2022;8:404-11. [Crossref] [PubMed]
- Kadri Y, Phan M, Bambace N, et al. Donor Age and Non-Relapse Mortality: Study of Their Association after HLA-Matched Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndrome. Curr Oncol 2022;29:5955-62. [Crossref] [PubMed]
- Kröger N, Zabelina T, de Wreede L, et al. Allogeneic stem cell transplantation for older advanced MDS patients: improved survival with young unrelated donor in comparison with HLA-identical siblings. Leukemia 2013;27:604-9. [Crossref] [PubMed]
- Abid MB, Estrada-Merly N, Zhang MJ, et al. Younger Matched Unrelated Donors Confer Decreased Relapse Risk Compared to Older Sibling Donors in Older Patients with B Cell Acute Lymphoblastic Leukemia Undergoing Allogeneic Hematopoietic Cell Transplantation. Transplant Cell Ther 2023;29:611-8. [Crossref] [PubMed]
- Fleischhauer K, Tran TH, Meisel R, et al. Donor Selection for Allogeneic Hematopoietic Cell Transplantation. Dtsch Arztebl Int 2023;120:261-8. [Crossref] [PubMed]
- Kao RH, Li CC, Shaw CK, et al. Correlation between characteristics of unrelated bone marrow donor and cell density of total nucleated cell in bone marrow harvest. Int J Hematol 2009;89:227-30. [Crossref] [PubMed]
- Mamo T, Sumstad D, DeFor TE, et al. Harvest Quality, Nucleated Cell Dose and Clinical Outcomes in Bone Marrow Transplantation: A Retrospective Study. Transplant Cell Ther 2023;29:638.e1-8. [Crossref] [PubMed]
- Streck BP, Naufal G, Carrum G, et al. Demographic and Clinical Donor Characteristics as Predictors of Total Nucleated Cell Concentrations in Harvested Marrow Products. Transplant Cell Ther 2021;27:785.e1-6. [Crossref] [PubMed]
- Rimondo A, Bramanti S, Crocchiolo R, et al. Bone marrow donor-related variables associated with harvest outcome in HLA-haploidentical transplantation with postinfusion cyclophosphamide. Vox Sang 2016;111:93-100. [Crossref] [PubMed]
- Anthias C, Billen A, Arkwright R, et al. Harvests from bone marrow donors who weigh less than their recipients are associated with a significantly increased probability of a suboptimal harvest yield. Transfusion 2016;56:1052-7. [Crossref] [PubMed]
- Rennert W, Cormier K, Sprott S. The Donor – Recipient Weight Ratio is a Reliable Marker for Cell Yield in Hematopoietic Stem Cell Donations. OBM Transplantation 2021;5: [Crossref]
- Rennert W, Sobh L, Cormier K, et al. The impact of donor total estimated blood volume on nucleated cell yield in bone marrow harvests for hematopoietic stem cell transplantation. Transfusion 2021;61:1533-41. [Crossref] [PubMed]
- Coombs CC, Zehir A, Devlin SM, et al. Therapy-Related Clonal Hematopoiesis in Patients with Non-hematologic Cancers Is Common and Associated with Adverse Clinical Outcomes. Cell Stem Cell 2017;21:374-382.e4. [Crossref] [PubMed]
- Chang E, Forsberg EC, Wu J, et al. Cholinergic activation of hematopoietic stem cells: role in tobacco-related disease? Vasc Med 2010;15:375-85. [Crossref] [PubMed]
- Suwa T, Hogg JC, Vincent R, et al. Ambient air particulates stimulate alveolar macrophages of smokers to promote differentiation of myeloid precursor cells. Exp Lung Res 2002;28:1-18. [Crossref] [PubMed]
- Luis TC, Barkas N, Carrelha J, et al. Perivascular niche cells sense thrombocytopenia and activate hematopoietic stem cells in an IL-1 dependent manner. Nat Commun 2023;14:6062. [Crossref] [PubMed]
- Bello AB, Park H, Lee SH. Current approaches in biomaterial-based hematopoietic stem cell niches. Acta Biomater 2018;72:1-15. [Crossref] [PubMed]
- Sánchez-Lanzas R, Kalampalika F, Ganuza M. Diversity in the bone marrow niche: Classic and novel strategies to uncover niche composition. Br J Haematol 2022;199:647-64. [Crossref] [PubMed]
- Fujiwara SI, Ikeda K, Kino S, et al. Clinical significance of autologous blood transfusions in bone marrow harvest from unrelated donors. Int J Hematol 2020;111:833-9. [Crossref] [PubMed]
- Lysák D, Hejretová L, Hrabětová M, et al. Should We Stop Collecting the Preoperative Autologous Blood before Bone Marrow Harvest? J Clin Med 2021;10:2134. [Crossref] [PubMed]
- Farhadfar N, Murthy HS, Logan BR, et al. Impact of autologous blood transfusion after bone marrow harvest on unrelated donor’s health and outcome: a CIBMTR analysis. Bone Marrow Transplant 2020;55:2121-31. Erratum in: Bone Marrow Transplant 2021;56:522. [Crossref] [PubMed]
- Teofili L, Valentini CG, Bianchi M, et al. Preoperative autologous blood donation in adult bone marrow donors: reappraisal of a single-centre experience. Vox Sang 2019;114:762-8. [Crossref] [PubMed]
- Kim-Wanner SZ, Luxembourg B, Schmidt AH, et al. Introduction of principles of blood management to healthy donor bone marrow harvesting. Vox Sang 2020;115:802-12. [Crossref] [PubMed]
- Arora K, Kelley J, Martinez F, et al. Preoperative autologous blood collection before bone marrow harvests in haploidentical related donors: is it justified? Transfusion 2018;58:1618-25. [Crossref] [PubMed]
- Rennert WP, Smith M J, Cormier KA, et al. Supportive Care of Hematopoietic Stem Cell Donors. Clin Hematol Int 2024;6:43-50. [Crossref] [PubMed]
- Epah J, Spohn G, Preiß K, et al. Small volume bone marrow aspirates with high progenitor cell concentrations maximize cell therapy dose manufacture and substantially reduce donor hemoglobin loss. BMC Med 2023;21:360. [Crossref] [PubMed]
- Bacigalupo A, Tong J, Podesta M, et al. Bone marrow harvest for marrow transplantation: effect of multiple small (2 ml) or large (20 ml) aspirates. Bone Marrow Transplant 1992;9:467-70. [PubMed]
- Oliver K, Awan T, Bayes M. Single- Versus Multiple-Site Harvesting Techniques for Bone Marrow Concentrate: Evaluation of Aspirate Quality and Pain. Orthop J Sports Med 2017;5:2325967117724398. [Crossref] [PubMed]
- Witt V, Pichler H, Fritsch G, et al. Multiple small versus few large amount aspirations for bone marrow harvesting in autologous and allogeneic bone marrow transplantation. Transfus Apher Sci 2016;55:221-4. [Crossref] [PubMed]
- Lannert H, Able T, Becker S, et al. Optimizing BM harvesting from normal adult donors. Bone Marrow Transplant 2008;42:443-7. [Crossref] [PubMed]
- Wang TF, Chu SC, Chen SH, et al. The effect of different harvest strategies on the nucleated cell yields of bone marrow collection. Biol Blood Marrow Transplant 2011;17:351-5. [Crossref] [PubMed]
Cite this article as: Rennert W. Optimizing total nucleated cell yield in bone marrow harvests for hematopoietic stem cell transplantation. Ann Blood 2024;9:29.


