Plasma fractionation in China: progress and challenges
Currently, China is one of the largest consumers and manufacturers in the global plasma-derived products market. This regional market is growing at a rapid pace with the increasing demands of a huge population of more than 1.3 billion.
There are two independent blood service systems in China: the voluntary non-remunerated whole blood banking system and the commercial source plasma collection system. Whole blood and apheresis platelets collected from the voluntary non-remunerated whole blood banking system are used directly in clinical transfusion, while commercial source plasma is fractionated into plasma-derived products. There is no non-paid plasma donor in China. Plasmapheresis centers were state-owned, just like voluntary non-remunerated blood centers, before 2006. Nevertheless, the “Working Plan on Transformation of Plasmapheresis Centers” was issued by nine national ministries and commissions in 2006 (1), as management methods changed when ownership was transferred from local governments to plasma fractionation enterprises.
Legislative framework and management system
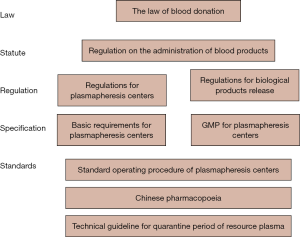
The Chinese blood donation law established in 1998 is the only law pertaining to blood donation and clinical transfusion and was a true milestone for the Chinese blood services system, followed by the issuance of a series of regulations and standards that have become the guiding principles to ensure plasma adequacy and safety (Figure 1). They set requirements on plasma donation sessions and donor selection criteria, and they established quality standards for plasma collection, storage and transportation.
The National Health and Family Planning Commission of the PRC (previously the MOH) is the competent authority for plasma collection management. The China Food and Drug Administration (CFDA) is responsible for fractionation and sales. The national regulatory framework is continuously changing in order to keep up with the evolving domestic needs. Plasma fractionation enterprises have taken charge of the quality of both plasma and its products.
The set up of commercial collection entities by plasma manufacturers should be in accordance with local government plans, strictly meet the relevant standards and requirements, and pass inspection for approval. All of the collection entities were established in counties with donors restricted to local residents.
National and regional supervisory visits or inspections were organized periodically as a sustainable mechanism to monitor blood services management. The subjects of inspection included policy implementation, quality management, improvement of services, and focusing on processes to outcomes. Covering “vein-to-vein”, these supervisory visits greatly facilitated improvements to blood safety and provided an effective method of surveillance and continuous advocacy for blood safety (2).
Plasma collection
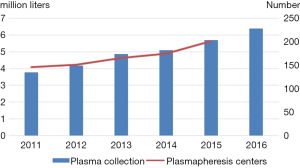
There were 201 commercial plasmapheresis centers by the end of 2015, which contributed about 6 million liters of source plasma for the manufacture of plasma-derived products (3). Plasma collection showed a steady trend of linear growth from 2011 to 2016 (3-6). It is more significant that registered donors maintained rapid growth over the past five years, although there has been a small increase in the number of plasmapheresis centers (Figure 2).
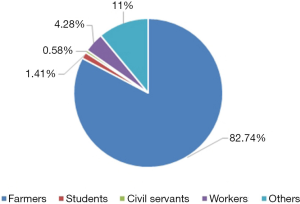
The distribution of donors across China as a whole (all donors were paid) consisted mainly of farmers, accounting for 82.74% of the total, with the proportion of workers, civil servants and students being extremely low (Figure 3).
Stations could only collect the plasma of certain people in their defined areas, and the government set up a strict standard for donor selection. In order to maintain donor safety, a most rigorous plasma collection frequency has been implemented, which is, namely, at least 14 days before the next donation (7).
Plasma donors in China are tested for their hemoglobin concentration, ABO group, HBsAg, hepatitis C virus (HCV) antibodies, alanine aminotransferase (ALT), HIV-1 and HIV-2 antibodies, and antibodies to treponemal antigens. Donors with reactive results to transfusion-transmitted viruses are permanently deferred. All of the plasma tests should have adopted nucleic acid testing (NAT) by the end of 2019 (8), which will further improve the safety of source plasma.
To ensure the safety of plasma, a quarantine period has been implemented since 2008 (9), which means that plasma must be stored for 90 days after collection, after which the donor’s blood is tested again. If the tests are negative, then the plasma collected 90 days prior may be released for production. This helps to avoid the threat of the “window period” and achieve a maximum level of plasma safety.
Technologies
In China, plasma derivatives were first produced in the 1960s by the Institute of Blood Transfusion and the Shanghai Institute of Biological Products using the Kistler/Nitschmann (10) and Cohn (11) plasma fractionation methods, respectively. Subsequently, “the cold ethanol precipitation method of plasma fractionation and its clinical application” passed the technical appraisal organized by the Chinese Ministry of Health in 1966 (12). Due to the economic situation and the need for combat readiness, the method of rivanol fractionation as a convenient, simple, and inexpensive procedure was widely adopted by most fractionators from the early 1970s, a procedure later annulled in 1995. Although plasma derivatives have been fractionated for about 50 years, large-scale production started relatively late (from the 1990s) and lagged in China compared with the developed countries. From the 1990s to the 2000s, most plasma fractionation plants used the cold ethanol fractionation precipitation procedure developed by Cohn (11) and modified by Kistler and Nitschman (10). In the meantime, a 90-day quarantine period (13), virus inactivation/removal technology (14) (including solvent/detergent treatment, dry heat, pasteurization, low pH incubation, nanofiltration, and so on; see Table 1), and the lot release of plasma-derivatives (13) were implemented to increase the safety against blood-borne viruses. Although the methods for plasma fractionation are getting more and more complicated (such as introducing the use of chromatography, depth filtration and ultrafiltration) after more than 50 years of development in China, the current core fractionation technology still critically relies on the cold ethanol precipitation process. It is worth noting that with the exception of just a small number of manufacturers producing intravenous immunoglobulin (IVIg) and coagulation factors by a process of integrated chromatography to cold ethanol precipitation (Table 1), most Chinese fractionators still use the method of cold ethanol precipitation to obtain albumin and IVIg. Progressively, a few manufacturers have introduced chromatographic technology to research and develop new protein therapeutics, such as obtaining antithrombin III from Cohn fraction IV by combining ion exchange chromatography with heparin affinity chromatography, isolating protein C from cryo-poor plasma using the combination of ion exchange chromatography with heparin affinity chromatography, and separating the von Willebrand factor from cryoprecipitate undergoing two ion exchange chromatographic processes.
Table 1
| Products | Technological trends | Virus inactivation/removal | |||
|---|---|---|---|---|---|
| Before 2000 | After 2000 | Before 1998 | After 1998 | ||
| IVIg | Ethanol precipitation | Ethanol precipitation + ion exchange chromatography | None | Low pH (pH 4.0) treatment + pasteurization or nanofiltration | |
| Factor VIII | Cryoprecipitate +acid precipitate +Al(OH)3 absorption | Cryoprecipitate + acid precipitate + Al(OH)3 absorption + ion exchange chromatography | None | Solvent-detergent + vapor heat or dry heat | |
IVIg, intravenous immunoglobulin.
Plasma fractionation
As a result of not having any requirements on good manufacturing practice for pharmaceuticals (GMP), more than 100 fractionators subsequently sprung up all over China. However, the production capacity of most fractionators was extremely small, at less than 10,000 L per year. At that time, only 3 plasma derivatives (albumin (20%), intramuscular immunoglobulin (IMIg) and fibrinogen concentrate) were available in China. According to the Regulations on the Administration of Blood Products issued in 1996 (15), there are strict requirements to be met, including donor screening, automated (not manual) plasmapheresis, the storage of collected plasma, and product manufacture and distribution steps, etc. Since 1998, the development of the Chinese plasma fractionation industry has been on the right track as a result of the implementation of the GMP principles in plasma product establishments to improve product quality, safety and supply. Meanwhile, the number of fractionators sharply declined from more than 100 to less than 30. Nowadays, the total designed production capacity (each batch of 2,500–5,000 L) is 12,000,000 L per year, however the total collected plasma is only approximately 7,000,000 L/year in China. More than 25 different protein products are available in developed countries, whereas only 12 plasma-derivatives can be extracted through large-scale fractionation of human plasma in China, including albumin, immunoglobulin (freeze-dried), hepatitis B immunoglobulin (freeze-dried), intravenous hepatitis B immunoglobulin (pH 4), intravenous hepatitis B immunoglobulin (pH 4, freeze-dried), IVIg, rabies immunoglobulin (freeze-dried), tetanus immunoglobulin (freeze-dried), coagulation factor VIII, fibrinogen, fibrin sealant and prothrombin complex.
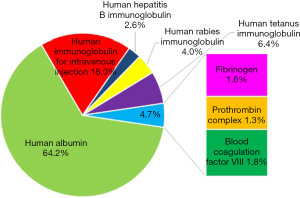
China is one of the most important parts of the global market by both volume and value. Albumin assumes the largest proportion (Figure 4), with imported albumin taking more than a half. Consumers in China use albumin for volume replacement therapy. Albumin is also prescribed in China for other conditions such as kidney diseases, cirrhosis, cancer, and others.
According to data on blood products from the lot release system of eight biological products verification authorities of the China’s State Food and Drug Administration, a total of 4,038 batches were issued in 2016 (Table 2).
Table 2
| Varieties | Batches |
|---|---|
| Human albumin | 2,680 |
| Human immunoglobulin for intravenous injection (pH 4) | 711 |
| Freeze-dried intravenous human immunoglobulin (pH 4) | 13 |
| Human normal immunoglobulin | 36 |
| Human rabies immunoglobulin | 46 |
| Human tetanus immunoglobulin | 65 |
| Human hepatitis B immunoglobulin | 39 |
| Human intravenous hepatitis B immunoglobulin | 6 |
| Freeze-dried intravenous hepatitis B immunoglobulin | 1 |
| Fibrinogen | 169 |
| Prothrombin complex | 143 |
| Blood coagulation factor VIII | 129 |
| Total | 4,038 |
There were 3 kinds and 11 brands of plasma products in the Chinese Pharmacopoeia of 2015. The main product types and approval numbers of major plasma products facilities are shown in Table 3.
Table 3
| Product type | Hualan Biological | China National Biotec Group | Taibang Biological | Shuyang Pharmaceutical | Shanghai RAAS | Boya biopharmaceuticals |
|---|---|---|---|---|---|---|
| Albumin | √ | √ | √ | √ | √ | √ |
| Intravenous immunoglobulin | √ | √ | √ | √ | √ | √ |
| Freeze-dried intravenous immunoglobulin | √ | √ | √ | √ | ||
| Human immunoglobulin | √ | √ | √ | √ | √ | √ |
| Hepatitis B immunoglobulin | √ | √ | √ | √ | √ | √ |
| Intravenous hepatitis B immunoglobulin | √ | √ | √ | √ | ||
| Rabies immunoglobulin | √ | √ | √ | √ | √ | √ |
| Tetanus immunoglobulin | √ | √ | √ | √ | √ | |
| Factor VIII | √ | √ | √ | √ | ||
| Human fibrinogen | √ | √ | √ | √ | √ | |
| Human prothrombin complex | √ | √ | √ | √ |
√, with approval number.
Future and challenges
Working together, great progress has been made in the Chinese plasma fractionation industry over the past 50 years. Both blood and plasma-derived products are regarded as strategic resources in China.
However, problems still remain. While millions of liters of plasma were collected annually with steady increases, self-sufficiency and strategic independence of plasma-derived products has nonetheless not yet been achieved. Clinical demand for plasma-derived products is increasing. Thousands of liters of plasma are discarded as unfit for fractionation, a loss that is both economically and morally inexcusable. Shortages of plasma for fractionation have become the “bottleneck” restricting the development of the Chinese plasma fractionation industry. In addition, Chinese plasma fractionators should enhance the research and development of new protein therapeutics to utilize the limited and valuable plasma sources as much as possible. The situation might change when a new edition of the Regulations on the Administration of Blood Products is issued so as to lead plasma fractionation industry development in a more rational and accelerated way.
With increasing domestic market demand, China keeps finding ways of communicating and collaborating with the international market. Contact between Chinese and foreign fractionators has been more and more frequent due to an open and free trade environment in China. The Chinese Innovation Union of plasma fractionators and collectors was established in 2017. Representatives of foreign fractionators were invited to participate in the communications and discussions. However, constructive dialogue between Chinese and foreign regulatory agencies (and fractionators) is still necessary and should be fostered in a regular and official way.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Thierry Burnouf) for the series “Plasma Fractionation” published in Annals of Blood. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aob.2018.02.02). The series “Plasma Fractionation” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Health and Family Planning Commission of the PRC. Working Plan on Transformation of Plasmapheresis Centers. 2006. Available online: http://www.moh.gov.cn/mohyzs/s3589/200804/18671.shtml
- Wang Y, Wu Y, Chen Y, et al. The journey toward safer and optimized blood service in China: national strategy and progress. Transfusion 2016;56:3112-20. [Crossref] [PubMed]
- National Health and Family Planning Commission of the PRC. Explanation of Comments on Promoting the Healthy Development of Plasmapheresis Centers. 2016. Available online: http://www.moh.gov.cn/zwgk/jdjd/201612/766651a8b2fc4c4f9a816475f144e2af.shtml
- Blood Collection Data. MOH. Available online: http://www.nhfpc.gov.cn/zwgk/xycjxx/201405/a7487d6b2de3430a98d7bc254bc62cf2.shtml
- Zhu YM. Annual Report on Development of China's Blood Collection and Supply Industry. Social Sciences Academic Press. 2016.
- Promote the Comprehensive Development of Blood Management. Oral Presentation. 28th Regional Congress of the ISBT, Guangzhou, China.
- National Health and Family Planning Commission of the PRC. Provisions for plasma donation center management. 2008. Available online: http://www.nhfpc.gov.cn/zwgk/wlwl/200804/d3ee56e92e4b448a8cc16ff38692d4de.shtml
- National Health and Family Planning Commission of the PRC. Comments on Promoting the Healthy Development of Plasmapheresis Centers. 2016. Available online: http://www.nhfpc.gov.cn/yzygj/s3590/201612/6a508ca0df3d43428a3cb6419afb89d8.shtml
- CFDA. Notification of the plasma quarantine period for the production of blood products. 2007. Available online: http://www.sda.gov.cn/WS01/CL0844/10732.html
- Kistler P, Nitschmann H. Large scale production of human plasma fractions. Eight years experience with the alcohol fractionation procedure of Nitschmann, Kistler and Lergier. Vox Sang 1962;7:414-24. [Crossref] [PubMed]
- Cohn EJ, Strong LE, Hughes WL, et al. Preparation and Properties of Serum and Plasma Proteins. IV. A System for the Separation into Fractions of the Protein and Lipoprotein Components of Biological Tissues and Fluids. J Am Chem Soc 1946;68:459-75. [Crossref] [PubMed]
- Liu W, Li C, Wang Z, et al. Chapter 17: The isolation and purification of blood plasma derivatives. In: Yang C, Liu J, Zhao T. editors. Chinese Transfusion Medicine, 1st ed. Beijing: People’s Medical Publishing House, 2017:294-304.
- CFDA. China intensifies safety supervision on blood products. 2007. Available online: http://www.sfda.gov.cn/WS01/CL0205/15204.html
- CFDA. Guidelines for technical methods and validation of virus removal/inactivation in blood products. 2002. Available online: http://www.sfda.gov.cn/WS01/CL0058/9330.html
- The State Council of the People’s Republic of China. Regulations on the administration of blood products. The State Council of the People's Republic of China. 1996. Available online: http://www.sfda.gov.cn/WS01/CL0056/10774.html
Cite this article as: Wang Y, Wang Z, Liu B, Huang X, Li W, Li C. Plasma fractionation in China: progress and challenges. Ann Blood 2018;3:15.